Somapacitan (LYS/CYS) is a recombinant long-acting growth hormone analogue designed for once-weekly treatment of growth hormone deficiency (GHD) in adults and children. It is a modified human growth hormone (rhGH) with chemical conjugation—most likely polyethylene glycol (PEG)—at specific lysine (LYS) and cysteine (CYS) residues to prolong its half-life and sustain biological activity. This modification impacts pharmacokinetics, enabling less frequent dosing while preserving the native growth-promoting functions such as stimulating growth, metabolism, and acting via the growth hormone receptor (GHR).
Appearance:
- White to off-white lyophilized powder; upon reconstitution, forms a clear, colorless solution.
Source:
- Produced by recombinant DNA technology in systems like E. coli or mammalian cells, purified for pharmaceutical formulation.
Molecular Weight:
- Higher than native rhGH (~22 kDa) due to PEG or similar modifications; exact weight depends on conjugate size.
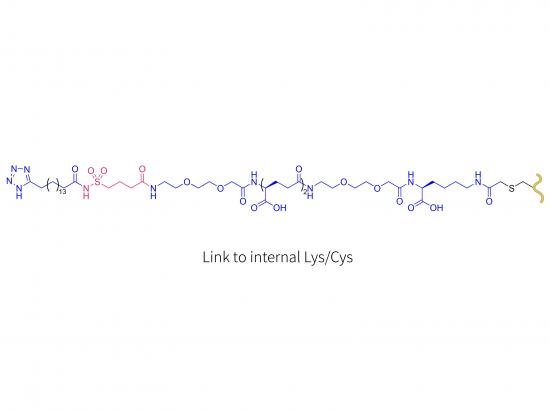
Structure:
- Human growth hormone amino acid sequence chemically modified at defined lysine and cysteine residues with PEG or another moiety; modification site critical for long-acting profile.
Biological Activity:
-
Activates GHR signaling pathways
-
Promotes bone growth, lean mass increase, fat metabolism
-
Modified sites enhance half-life, reducing injection frequency
Purity and Microbial Contamination:
-
Purity: ≥98% by HPLC, SDS-PAGE
-
Must meet sterility, bioburden, and endotoxin limits (LAL assay)
Identity and Quality Control:
-
Mass spectrometry confirms molecular weight and modification
-
Amino acid analysis and peptide mapping verify sequence and modification site
-
HPLC and SDS-PAGE assess purity and aggregation
-
Bioactivity assays confirm growth-promoting function
-
Immunochemical assays evaluate receptor binding
Shelf Life and Storage
| Feature | Description |
|---|---|
| Shelf Life | Typically 2–3 years from manufacturing (see official prescribing info) |
| Storage | Store refrigerated at 2–8°C; protect from light; do not freeze; use reconstituted solutions promptly as prescribed |
Applications:
-
Treatment of growth hormone deficiency in adults and children
-
Research into sustained release GH analogues and receptor interactions
Key Characteristics:
-
Recombinant human GH derivative
-
PEG or chemically modified at specific LYS/CYS residues to prolong half-life
-
Maintains biologic function while enabling once-weekly dosing
-
Pharmaceutical-grade lyophilized formulation
Research and Information Sources:
-
Regulatory approvals: FDA, EMA websites for official documents
-
Clinical trials: PubMed, clinicaltrials.gov for efficacy and safety data
-
PEGylation of proteins: Literature on PEGylated GH and other biotherapeutics
-
Patents: Google Patents and USPTO for manufacturing and structural details
-
Keywords for literature search: “Somapacitan mechanism of action,” “PEGylated growth hormone,” “Somapacitan clinical trials,” “growth hormone receptor binding affinity,” “recombinant human growth hormone manufacturing”
Reviews
There are no reviews yet.