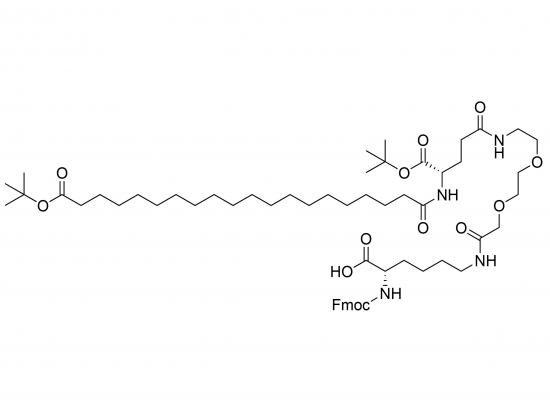
Fmoc-Lys(tBuO-Ara-Glu(AEEA)-OtBu)-OH is a complex, synthetically derived amino acid building block used in solid-phase peptide synthesis (SPPS). It features a modified lysine residue with a branched side chain functionalized by a tert-butyl ester-protected arabinogalactan (tBuO-Ara) linked to a tert-butyl ester-protected glutamic acid (Glu(OtBu)) residue, further extended by an AEEA (2-[2-(2-aminoethoxy)ethoxy]acetic acid) spacer. The Fmoc group protects the N-terminus for controlled SPPS deprotection. This intricate side chain enhances water solubility and biocompatibility and may improve pharmacokinetic properties. The AEEA spacer reduces steric hindrance, while tBu protecting groups provide orthogonality during synthesis. This building block is valuable for generating complex glycosylated or PEGylated peptides in applications including drug delivery, diagnostics, and biomaterials design.
Appearance
- White to off-white solid powder.
Source
- Synthetically produced through chemical synthesis, not naturally occurring.
Available from specialized chemical suppliers focusing on modified amino acids and peptide synthesis reagents.
Molecular Weight and Structure
-
The molecular weight is high, generally in the range of 900–1200 g/mol or more, depending on the arabinogalactan fragment size.
-
Composed of:
-
Fmoc (9-fluorenylmethoxycarbonyl) protecting group at N-terminus
-
Lysine core
-
tert-Butyl ester-protected arabinogalactan (tBuO-Ara) side chain
-
tert-Butyl ester-protected glutamic acid (Glu(OtBu))
-
AEEA spacer (2-[2-(2-aminoethoxy)ethoxy]acetic acid)
-
-
Due to structural complexity, precise structure diagrams can be found by searching related building blocks or contacting suppliers.
Biological Activity
-
Likely biologically inert by itself.
-
Biological effects depend on the peptide context and the attached carbohydrate/PEG-like moieties.
-
Arabinogalactan fragment may bind lectins or carbohydrate-binding proteins.
-
AEEA spacer enhances solubility and reduces steric hindrance in peptides.
Purity and Microbial Contamination
-
Purity typically ≥90%, assessed by HPLC; due to complexity, purity may be lower than simpler amino acids.
-
Microbial contamination should be minimal or absent, especially for biological use.
-
Suppliers often provide Certificates of Analysis (COAs) including bacterial endotoxin (LAL assay) and sterility tests.
Identity and Quality Control
-
Confirmed by:
-
High-resolution mass spectrometry (HRMS) for molecular weight verification
-
Nuclear Magnetic Resonance (¹H NMR, ¹³C NMR) despite complexity
-
Infrared (IR) spectroscopy for functional group presence
-
-
Quality controls include:
-
HPLC purity analysis
-
Amino acid analysis post-hydrolysis confirming Lys and Glu residues
-
Carbohydrate analysis verifying arabinogalactan presence
-
Shelf Life and Storage
| Feature | Description |
|---|---|
| Shelf Life | Likely shorter than simpler amino acids, approximately 6 months to 1 year. Consult supply COA. |
| Storage | Store at −20°C or below, in airtight container under inert atmosphere (argon or nitrogen). Protect from moisture and light. Avoid repeated freeze-thaw cycles. Desiccation important. |
Application
-
Solid-Phase Peptide Synthesis (SPPS) building block for branched, glycosylated, or PEGylated peptides
-
Drug delivery: improves solubility, biocompatibility, and targeting
-
Biomaterials: design of peptide-based materials with complex structures and functions
-
Diagnostics: peptides for imaging or biological target detection
Key Characteristics
-
Fmoc protection enables base-labile N-terminal deprotection during SPPS
-
Complex branched side chain with carbohydrate (arabinogalactan) and PEG-like (AEEA) properties
-
tert-Butyl (tBu) protecting groups offer acid-labile protection of functional groups
-
Enhances water solubility and reduces steric hindrance
-
Versatile for conjugation with other amino acids and peptide sequences
Citation
-
Relevant literature may be located by searching:
-
“Fmoc-Lys(modified)-OH” + “peptide synthesis” + “glycosylation” or “PEGylation”
-
“arabinogalactan peptide conjugate” or “PEGylated peptide synthesis”
-
“AEEA spacer peptide synthesis”
-
Supplier technical data sheets for modified amino acids
-
Chemical databases such as Reaxys, SciFinder for fragment-based searches
-
“solid phase peptide synthesis glycosylated peptide” or “PEGylated peptide”
-
“arabinogalactan drug delivery”
-
“PEG-like amino acid peptide”
-
“hydrophilic amino acid peptide”
-
-
Google Scholar: Keywords like “Fmoc lysine arabinogalactan peptide,” “PEGylated peptide synthesis,” “AEEA spacer peptide”
-
Since direct publications on this exact compound may be limited, consult related building block studies and review proprietary supplier data for deeper insight.
Reviews
There are no reviews yet.