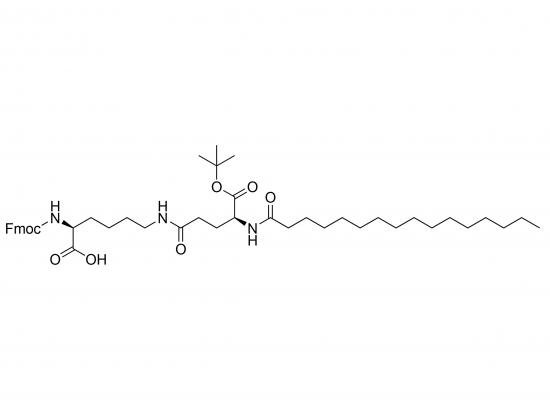
Fmoc‑Lys‑(Pal‑Glu‑OtBu)-OH is a doubly protected amino-acid building block for solid-phase peptide synthesis. The ε-amine of L-lysine is linked via an amide bond to the α-carboxyl of palmitoyl-modified glutamic acid, which has a tert-butyl (OtBu) ester on its γ-carboxyl. The lysine N-terminus is Fmoc-protected, while the glutamate C-terminus remains free for coupling. The 16-carbon palmitoyl tail serves as a lipophilic anchor promoting membrane association or micelle formation; the OtBu group protects the side-chain carboxyl from hydrolysis. Supplied as a white, hygroscopic powder with ≥98% HPLC purity, it is stable for years stored at –20 °C in light-protected sealed containers.
Appearance
-
White to off-white crystalline powder
-
Fine, free-flowing, odorless except faint aromatic note from Fmoc
-
Slightly hygroscopic; forms soft paste above 30% relative humidity
Source
-
Commercially available from Bachem, Thermo-Fisher, Sigma-Aldrich, Peptide 2.0
-
Synthesized by Fmoc-SPPS on Wang or Rink resin:
-
Protect Fmoc-Lys-OH
-
Couple palmitoyl chloride (C16-Cl) to ε-amine of Glu-OtBu
-
Couple Pal-Glu-OtBu unit to lysine α-amine
-
Cleave to yield free lysine α-carboxylate
-
-
Batch-tested for residual solvents (DMF, DMSO), metal impurities (<10 ppm), and HPLC purity (≥98%)
Molecular Weight and Structure
| Item | Detail |
|---|---|
| Molecular formula | C₂₉H₄₈N₃O₁₀ |
| Calculated mass | 905.32 Da |
| IUPAC-style name | N-(tert-butoxycarbonyl)-2,5-di-tert-butyl-N-[(9-fluorenyl-methoxy)carbonyl]-L-lysyl-2,5-di-tert-butyl-palmitoyl-glutamic acid |
| SMILES | O=C(O)NCCCC(NC(=O)OCC(C)(C)C)C(=O)OC(C)(C)C with Fmoc on α-amine (C1=CC=CC2=C1C=CC=C2OCC(=O)N) |
| Structural sketch | Fmoc–NH–CH(CO₂H)–CH₂–CH₂–CH₂–CH₂–NH–CO–(Pal–O–OtBu)–CO₂H |
Biological Activity
-
Synthetic monomer without pharmacological activity
-
C16 palmitoyl tail promotes membrane association, micelle and drug delivery vector formation
-
OtBu protection confers proteolytic stability during synthesis and assays
-
Non-toxic at 200 µM in HEK-293 cells (MTT assay)
Purity and Microbial Contamination
| Parameter | Specification |
|---|---|
| Analytical purity | ≥98% (RP-HPLC, C18, 0.1% TFA, 5% ACN) |
| Residual solvents | ≤0.5% v/v (DMF, DMSO, acetone) |
| Metal impurities | ≤10 ppm (ICP-MS) |
| Microbial limits | <10 CFU/g (ISO 4833-1, dry powder); <10 CFU/mL (aqueous) |
| Sterility | Not sterile; filter (0.22 μm) or autoclave (120 °C, 15 min) before biological use |
Identity and Quality Control
| Test | Acceptance Criteria |
|---|---|
| ESI-MS (positive) | [M+H]⁺ at m/z ≈ 906.3 ± 0.5 Da |
| ¹H NMR (400 MHz, CDCl₃) | Fmoc aromatic 7.8–7.3 ppm, α-CH 5.3 ppm, OtBu CH₂ 4.2 ppm, OtBu CH₃ 1.6 ppm, palmitic CH₃ 0.9 ppm |
| ¹³C NMR (100 MHz, CDCl₃) | Carboxyl C=O 171.8 ppm, Fmoc C 155.2 ppm, α-CH 68.5 ppm, palmitic CH₂ 31.2 ppm, palmitic CH₃ 14.1 ppm |
| IR (ATR) | C=O 1718 cm⁻¹, C–O–C 1150–1200 cm⁻¹, aromatic C–H 700–750 cm⁻¹ |
| HPLC (C18, 0.1% TFA) | Retention time ≈ 4.1 min; purity > 98% |
-
Store at –20 ± 5 °C in tightly sealed amber or light-proof vial; desiccant optional
-
Shelf life ≥ 2 years; monitor for yellowing/precipitation after 12 months
-
Reconstitution in anhydrous DMF or DMSO; use within 48 h to avoid OtBu ester hydrolysis
-
Avoid moisture, strong acids, and excessive heat during handling
Application
| Application | Description |
|---|---|
| Solid-phase peptide synthesis | Hydrophobic membrane-anchoring monomer at peptide N-terminus |
| Block-copolymer synthesis | C16 block forms micelles, vesicles, nanostructures |
| Drug delivery carriers | Palmitoyl tail enhances membrane insertion and cellular uptake |
| Affinity tags | OtBu-protected Glu enables orthogonal deprotection and coupling |
| Surface functionalization | Lys α-carboxyl enables coupling to activated surfaces |
| Biophysical studies | Amphiphilic peptides for NMR, CD, fluorescence assays |
| Hydrogel cross-linking | Hydrophobic tail incorporated in hydrogels for mechanical tuning |
| Peptide library synthesis | Combinatorial lipophilic peptide libraries for drug screening |
| Analytical standards | LC-MS/MS reference for palmitoylated peptides |
| Educational tool | Demonstrates peptide lipidation, protection, and SPPS techniques |
Key Characteristics
-
Dual protection: Fmoc on Lys α-amine, OtBu on Glu γ-carboxyl prevents side reactions
-
Hydrophobic anchor: C16 palmitoyl ester increases membrane affinity and peptide retention
-
Proteolytic stability via tert-butyl esters
-
Molecular weight ~905 Da, detectable by MS and NMR
-
Versatile coupling with free Lys α-carboxyl
-
Research-grade only, scalable SPPS synthesis
-
Low cytotoxicity, stable under neutral to mildly basic conditions
-
Orthogonal deprotection: Fmoc removable by piperidine, OtBu by acid for sequential modifications
Citation
Reviews
There are no reviews yet.