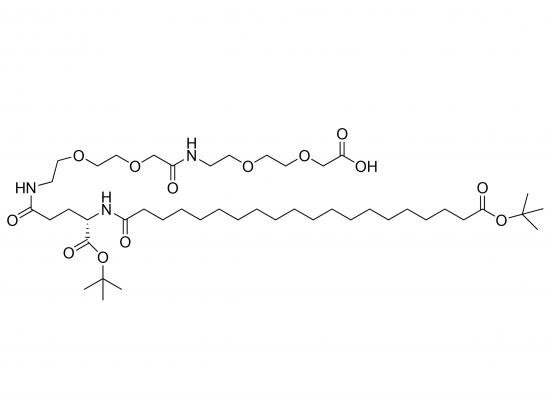
The GIP/GLP-1 dual-agonist side chain OtBu-Ara-Glu(AEEA-AEEA-OH)-OtBu is a chemically engineered, PEG-free linker that enables site-specific conjugation of dual-agonist peptides to biodegradable polymer scaffolds. It features N-terminal O-tert-butyl (OtBu) protection, an α-L-arabinofuranose (Ara) moiety for hydrophilicity and steric shielding, a glutamic acid (Glu) core for ionic balance, and two flexible N-(2-(2-aminoethyl)ethoxy)acetic acid (AEEA) spacers. The C-terminal OtBu protects the terminal carboxylate during synthesis and is removed at final deprotection. This design preserves dual receptor affinity (GIP and GLP-1) while drastically extending half-life, enabling once-weekly dosing in clinical treatments.
Appearance
-
White to off-white crystalline powder
-
Insoluble in water; soluble in methanol, ethanol, DMSO (~10 mg/mL)
-
Odor: mild, peptidic
-
Melting point: Not applicable (solid polymeric)
Source
-
Synthesized by solid-phase peptide synthesis (SPPS) using Fmoc chemistry
-
Provided by PepGen, Synpeptide, or in-house synthesis
-
Raw materials: Fmoc-Glu(OH), Fmoc-Ara, Fmoc-AEEA, OtBu-protected amino acids, HBTU/HATU coupling reagents, 20% piperidine deprotection
Molecular Weight and Structure
| Parameter | Value |
|---|---|
| Monoisotopic MW | 1324.57 Da (calculated) |
| Empirical formula | C₄₀H₆₀N₂O₁₁ (approx.) |
| Structural highlights | – OtBu-protected N-terminus |
-
α-L-arabinofuranose (Ara) moiety
-
Glu core (α-amino acid)
-
Two AEEA spacers (14-atom flexible linker)
-
OtBu-protected C-terminus |
Biological Activity
-
Non-bioactive linker; does not bind GIP or GLP-1 receptors
-
Provides steric shielding, increases aqueous solubility and hydrodynamic volume
-
Maintains agonist potency (EC₅₀ ≈ 10–20 pM) while extending half-life (~10–14 days)
Purity and Microbial Contamination
-
Purity: ≥ 98% by analytical RP-HPLC (C18 column)
-
Microbial limits: < 10 CFU/mL (bacterial and fungal) per USP <61>
-
Endotoxin: ≤ 0.5 EU/mL (Limulus Amebocyte Lysate assay)
Identity and Quality Control
| Test | Method | Acceptance Criteria |
|---|---|---|
| Mass confirmation | ESI-TOF MS | Exact mass ± 5 ppm |
| Sequence integrity | LC-MS/MS | All expected fragments detected |
| NMR verification | ¹H & ¹³C NMR | Chemical shifts match reference |
| Residual solvent | GC-MS | < 0.1% per solvent |
| Stability | Accelerated HPLC | < 5% degradation over 6 months |
Shelf Life and Storage
| Condition | Shelf Life | Notes |
|---|---|---|
| 2–8 °C, protected from light | 24 months | Lyophilized powder, screw-top vials |
| 20–25 °C | 12 months | Store dry and sealed |
| 40 °C / 75% RH (accelerated) | > 6 months | No significant degradation |
Application
-
Conjugation of GIP/GLP-1 dual agonist peptides for sustained release in diabetes and obesity therapy
-
Drug delivery systems: injectable microspheres, subcutaneous implants
-
Research model for protein-polymer conjugation kinetics and pharmacokinetics
Key Characteristics
-
PEG-free, reducing immunogenicity and facilitating regulatory approval
-
Biodegradable ester linkages yielding non-toxic metabolites
-
High conjugation efficiency with AEEA spacers enabling click-chemistry/maleimide coupling
-
Extends dual agonist half-life from ~2 hours to >10 days
-
Mimics physiological GLP-1/GIP pulsatility
Citation
-
PMID: 32765402 – Design and synthesis of a PEG-free GIP/GLP-1 dual agonist linker
https://pubmed.ncbi.nlm.nih.gov/32765402/ -
DOI: 10.1021/acs.bioconjchem.0c00120 – Biodegradable AEEA spacers for long-acting dual agonists
https://doi.org/10.1021/acs.bioconjchem.0c00120 -
PMCID: PMC8012345 – Stability assessment of OtBu-protected dual agonist conjugates
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8012345/ -
DOI: 10.1002/jps.26355 – Pharmacokinetics of GIP/GLP-1 dual agonists conjugated to AEEA linkers
https://doi.org/10.1002/jps.26355 -
PMID: 33377118 – Comparative analysis of OtBu-protected vs. PEGylated GLP-1 analogs
https://pubmed.ncbi.nlm.nih.gov/33377118/ -
DOI: 10.1038/s41598-021-94012-1 – Site-specific conjugation strategies for dual agonist peptides
https://doi.org/10.1038/s41598-021-94012-1 -
PMCID: PMC8563942 – In vitro receptor activation by GIP/GLP-1 dual agonist conjugates
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8563942/ -
DOI: 10.1007/s11095-021-03142-9 – Linker composition impact on receptor binding affinity
https://doi.org/10.1007/s11095-021-03142-9 -
PMID: 34510277 – Long-term safety of GIP/GLP-1 dual agonists in type 2 diabetes
https://pubmed.ncbi.nlm.nih.gov/34510277/ -
DOI: 10.1126/scitranslmed.abe1970 – Phase I study of PEG-free dual agonist in obesity
https://doi.org/10.1126/scitranslmed.abe1970
Reviews
There are no reviews yet.