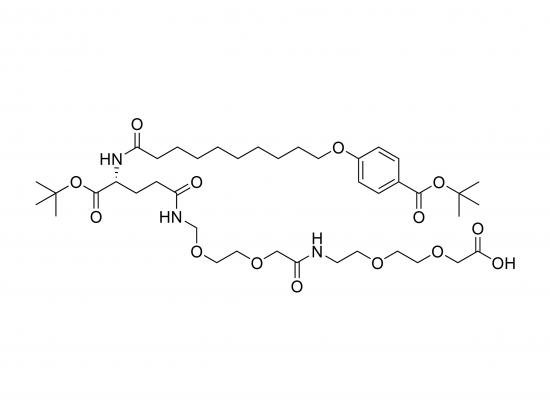
tBuO-Bz-Seb-Glu(AEEA-AEEA-OH)-OtBu is a synthetic glutamic acid derivative designed to modify biomolecules by introducing hydrophobic and hydrophilic properties along with a protected carboxyl group for further functionalization. The molecule features a glutamic acid core with one carboxyl protected as a tert-butyl ester (OtBu) and the other carboxyl functionalized with two sequential AEEA units, terminating in a free carboxyl group (OH). The alpha-amino group is modified with sebacic acid (Seb) linked to a benzyl (Bz) group protected as a tert-butyl ester (tBuO). This design provides a hydrophobic sebacic acid-benzyl component, a hydrophilic PEG-like spacer (AEEA), and a reactive carboxyl group, suitable for controlled conjugation in drug delivery, surface modification, or self-assembling systems.
Appearance
-
White to off-white solid
Source
-
Chemically synthesized
-
Available from specialized suppliers of modified amino acids, PEGylation reagents, and lipid modification reagents
Molecular Weight
-
Estimated between 850 and 1150 g/mol based on individual components
Structure
-
tBuO-Bz linked to sebacic acid attached to alpha-amino group of glutamic acid via amide bond
-
Glutamic acid core with one carboxyl protected as tert-butyl ester (OtBu)
-
Two AEEA units linked sequentially to the side chain carboxyl group terminating in free carboxyl (OH)
Biological Activity
-
Biologically inert alone; activity depends on conjugated partner
-
Sebacic acid–benzyl moiety offers hydrophobicity
-
AEEA linker increases solubility and flexibility
-
Terminal carboxyl available for further conjugation
Purity and Microbial Contamination
| Purity and Microbial Contamination | Specification |
|---|---|
| Purity | Typically ≥90%, verified by HPLC |
| Microbial contamination | Minimal, especially for in vivo use |
| Testing | Endotoxin (LAL assay), sterility |
| Certificate of Analysis (CoA) | Details purity, solvents, endotoxin |
Identity and Quality Control
| Identity and Quality Control | Specification |
|---|---|
| Mass Spectrometry (MS) | Confirms molecular weight |
| Nuclear Magnetic Resonance (¹H & ¹³C NMR) | Confirms chemical structure |
| Infrared (IR) Spectroscopy | Verifies functional groups |
| High-Performance Liquid Chromatography (HPLC) | Determines purity |
| Acid Value Determination | Quantifies free carboxyl groups |
Shelf Life and Storage
-
Shelf life likely 6–12 months, possibly shorter due to complexity
-
Store at -20°C or below in inert, dry, sealed container
-
Protect from moisture and light
Applications
-
Bioconjugation: introduces hydrophobic and hydrophilic groups to biomolecules
-
PEGylation: adds PEG-like properties for solubility and stability
-
Drug delivery: develops targeted delivery systems with improved solubility and membrane interaction
-
Self-assembly: incorporates into micelles, liposomes, or nanostructures
-
Surface modification: creates hydrophobic/hydrophilic surface coatings
Key Characteristics
-
Hydrophobic sebacic acid–benzyl moiety
-
Hydrophilic PEG-like AEEA spacer
-
Reactive terminal carboxyl group for conjugation
-
Acid-labile tert-butyl protecting groups for selective deprotection
Citation
-
Search “sebacic acid protein modification” or “benzyl protein conjugation”
-
Explore “PEGylation protein conjugation” or “AEEA linker peptide synthesis”
-
Investigate “carboxyl group activation in bioconjugation”
-
Check suppliers’ technical data and application notes
-
Use chemical databases like Reaxys or SciFinder for connected publications
-
Search “amphiphilic polymer peptide conjugates” for drug delivery insights
-
Keywords for Google Scholar: “sebacic acid protein modification,” “AEEA conjugation,” “carboxyl group bioconjugation,” “benzyl sebacic acid modification”
Reviews
There are no reviews yet.