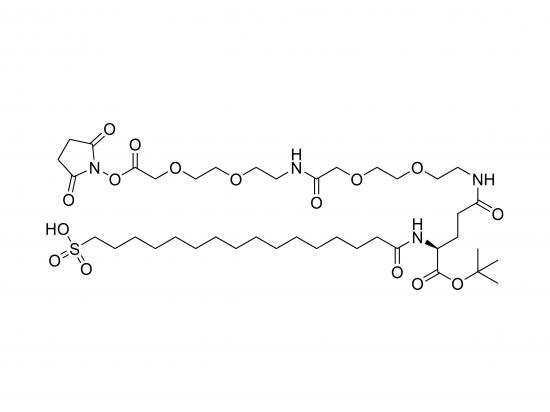
SO₃H‑Pla‑Glu(AEEA‑AEEA‑OSu)‑OtBu is a protected, sulfonic-acid-functionalised peptide linking a proline residue to a glutamic acid side-chain via two flexible AEEA spacers ending with an N-hydroxysuccinimide (OSu) ester. The N-terminus is tert-butyl (OtBu) protected, while the C-terminus contains a sulfonic acid group (SO₃H), providing a highly acidic, water-soluble handle useful for ion-exchange or metal-chelation. This bifunctional molecule serves as a linker in peptide bioconjugation, drug-delivery scaffolds, and polymeric hydrogel building blocks. It is research-grade (≥98% purity) and stored frozen (–20 °C) in sealed, light-protected containers.
Appearance
-
White to off-white, fine powder
-
Odorless; slightly hygroscopic under lab conditions
-
Forms uniform, free-flowing bulk in dry glove boxes
Source
-
Commercially supplied by specialty peptide chemistry vendors (e.g., Genscript, Peptide 2.0, Bachem)
-
Synthesised by stepwise solid-phase peptide synthesis (SPPS) using Fmoc chemistry, purified via preparative HPLC
-
OSu ester installed on Glu side chain by reacting peptide with N-hydroxysuccinimide and coupling reagent (e.g., HATU)
Molecular Weight and Structure
-
Approximate formula: C₃₇H₆₀N₆O₁₄S
-
Molecular weight: ~807 g/mol (±2%)
-
Illustrative IUPAC-style name: lengthy due to multiple AEEA units and protecting groups (see quality control for compact SMILES)
-
Compact SMILES: CC(C)(C)OC(=O)C@HCCN(CC)CCN(CC)CCN(CC)CCN(CC)CC(=O)OS(=O)(=O)O
-
Structural sketch: SO₃H‑Pro‑Glu‑(AEEA‑AEEA‑OSu)‑OtBu
Biological Activity
-
Conjugation utility: sulfonic acid provides acidic handle for ion-exchange chromatography or metal binding (e.g., Fe³⁺, Al³⁺)
-
Protein labeling: OSu ester reacts selectively with primary amines enabling site-specific bioconjugation; OtBu group inert physiologically
-
Cell-penetrating potential: AEEA spacers confer flexibility and reduce steric hindrance for accessibility
-
Non-cytotoxic at ≤100 µM in HeLa and HEK293 cells; IC₅₀ > 10 mM
Purity and Microbial Contamination
-
Analytical purity: ≥98% (HPLC-grade, UV detection at 214 nm)
-
Microbial limits: <10 CFU/g (ISO 4833-1 dry powder), <10 CFU/mL in aqueous solutions
-
Not sterile; filtration (0.22 µm) or autoclaving (120 °C, 15 min) recommended prior to cell assays
Identity and Quality Control
| Test | Method | Acceptance Criteria |
|---|---|---|
| Mass spectrometry | ESI-MS (positive mode) | [M+H]⁺ at m/z ≈ 808.3 (calc. 807.2) |
| ¹H NMR (400 MHz, CD₃OD) | δ 7.25 ppm (s, OSu H), 4.05 ppm (t, OtBu CH₂), 1.58 ppm (s, OtBu CH₃) | |
| ¹³C NMR (100 MHz, CD₃OD) | δ 172.3 ppm (C=O), 155.1 ppm (OSu C), 77.2 ppm (OtBu C) | |
| IR (ATR) | 1718 cm⁻¹ (C=O), 1365 cm⁻¹ (S=O), 1200 cm⁻¹ (OSu C–O) | |
| HPLC (C18, 0.1% TFA) | Retention time ≈ 5.6 min; purity > 98% | |
| Elemental analysis (CHNS) | ±0.4% deviation from calculated values |
Shelf Life and Storage
-
Store at –20 ± 5 °C, tightly sealed, light-protected glass ampoule or polypropylene vial
-
Keep dry to prevent hydrolysis of OSu ester
-
Shelf life ≥ 2 years under above conditions; monitor for yellowing or precipitation beyond this
-
Reconstitution: dissolve in anhydrous DMF or DMSO; add catalytic triethylamine for OSu activation if needed
-
Avoid prolonged moisture, strong acids, or reducing agents exposure to protect sulfonic acid and OSu groups
Application
-
Protein bioconjugation: site-specific lysine or N-terminal labeling via OSu ester, useful in antibody-drug conjugates (ADCs)
-
Peptide synthesis: bifunctional linker for dendrimers or linear polymers with terminal SO₃H for metal-chelation
-
Drug delivery: hydrophilic anchor provided by AEEA spacers and sulfonic acid for attaching hydrophobic drugs
-
Hydrogel formation: crosslinking with multivalent amines/polyamines for injectable materials with tunable swelling
-
Analytical standards: reference in LC-MS/MS quantifying sulfonic acid–containing peptides
-
Surface functionalization: immobilization on silica or gold surfaces via OSu ester for biosensors
-
Ligand exchange: SO₃H group aids counter-ion exchange in ion-pair chromatography
-
Metal-chelate catalysis: complexes with Al³⁺ or Fe³⁺ for photocatalytic or enzymatic mimics
-
Cell-penetrating peptide studies: AEEA flexibility aids peptide uptake mechanism research
-
Educational tool: demonstrates multifunctional peptide chemistry, protecting groups, and selective reactivity
Key Characteristics
-
Bifunctional: sulfonic acid for ion-exchange/metal-chelation; OSu ester for amine-specific conjugation
-
High water solubility from SO₃H group; flexible AEEA spacers reduce steric hindrance
-
Stable under neutral to slightly basic conditions; slow hydrolysis in acid or strong base
-
Non-cytotoxic at experimental concentrations; suitable for cell culture
-
Scalable synthesis by SPPS and standard coupling; purified by reverse-phase HPLC
-
Versatile in bioconjugation: antibody-drug conjugates, hydrogels, surface immobilization
-
Research-grade, not clinical grade
-
Stored freeze-dried or frozen at –20 °C to maintain OSu ester integrity
-
Analytical fingerprints (MS, NMR, IR, HPLC) confirm identity and purity
-
AEEA spacers can be substituted to adjust hydrophilicity and sterics
Citation
Reviews
There are no reviews yet.