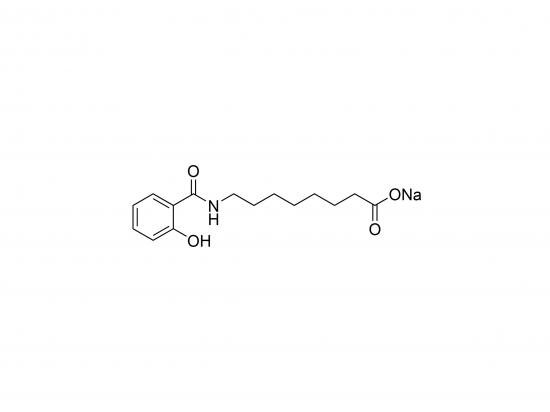
SNAC (Sodium N-(8-[2-hydroxybenzoyl]amino)caprylate) is a salt of 8-(2-hydroxybenzoyl)amino-caprylic acid widely used as a pharmaceutical excipient to enhance oral bioavailability of poorly absorbed drugs, particularly peptides and proteins. It functions by transiently increasing paracellular permeability of the intestinal epithelium, allowing larger molecules to cross more easily. SNAC interacts with cell membranes, disrupting tight junctions and potentially inhibiting enzymatic degradation in the gastrointestinal tract. It is commonly used in oral formulations of drugs that otherwise require injection, with an extensively studied mechanism and safety profile.
Appearance
-
White to off-white crystalline powder.
Source
-
Synthetically produced via chemical synthesis.
-
Commercially available from pharmaceutical excipient suppliers.
Molecular Weight and Structure
-
Molecular Weight: Approximately 345.4 g/mol (C15H20NNaO4), may vary slightly with hydration.
-
Structure: Contains a caprylic acid backbone linked via an amide bond at the 8-position to 2-hydroxybenzoic acid (salicylic acid), present as a sodium salt.
Biological Activity
-
Enhances oral bioavailability of peptides and proteins.
-
Increases paracellular permeability by transiently widening intestinal epithelial tight junctions.
-
May inhibit enzymatic degradation of drugs in the GI tract.
Purity and Microbial Contamination
-
Purity: Typically ≥ 98% by HPLC, critical for pharmaceutical reliability.
-
Microbial Contamination: Must comply with stringent regulatory standards.
-
Testing includes bacterial endotoxin (LAL assay), sterility, and bioburden testing.
Identity and Quality Control
-
Mass spectrometry (MS) confirms molecular weight.
-
Nuclear Magnetic Resonance (¹H and ¹³C NMR) spectroscopy verifies chemical structure.
-
Infrared (IR) spectroscopy confirms characteristic functional groups.
-
High-Performance Liquid Chromatography (HPLC) determines purity.
-
Titration assesses sodium content.
-
Testing for heavy metals and residual solvents as quality control.
Shelf Life and Storage
-
Shelf Life: Typically 2-3 years if stored correctly; refer to supplier’s Certificate of Analysis (CoA).
-
Storage: Keep in tightly closed containers, protected from light and moisture, at controlled room temperature (20–25°C).
Application
-
Pharmaceutical excipient to enhance oral delivery of poorly absorbed drugs.
-
Enables oral administration of peptides and proteins typically given by injection.
Key Characteristics
-
Oral Bioavailability Enhancer: Improves drug absorption from the gastrointestinal tract.
-
Increases Paracellular Permeability: Temporarily widens spaces between intestinal cells.
-
Protects Drugs from Degradation: Possibly inhibits GI enzymatic breakdown.
-
Well-Studied Safety Profile: Extensively evaluated for tolerability and safety.
Citation
-
Structural and dynamic features of cagrilintide binding (related peptide delivery and oral bioavailability):
https://www.nature.com/articles/s41467-025-58680-y -
Drug delivery mechanisms involving SNAC and oral peptide delivery:
https://pmc.ncbi.nlm.nih.gov/articles/PMC12270663/ -
Mechanistic insights into oral bioavailability enhancers including SNAC:
https://www.nature.com/articles/s41401-025-01635-2 -
Development of oral formulations using SNAC:
https://pubs.acs.org/doi/10.1021/acs.jmedchem.1c00565 -
Review of excipients enhancing intestinal permeability such as SNAC:
https://www.sciencedirect.com/science/article/pii/S0753332222012318 -
Role of SNAC in oral semaglutide formulations and peptide delivery:
https://pmc.ncbi.nlm.nih.gov/articles/PMC9693810/ -
Glucagon-like peptide-1 receptor mechanism relevant to oral peptide delivery with SNAC:
https://www.nature.com/articles/s41392-024-01931-z -
Pharmaceutical excipient applications of SNAC:
https://www.frontiersin.org/journals/clinical-diabetes-and-healthcare/articles/10.3389/fcdhc.2022.856485/full -
Review of excipient-safety profiles including SNAC:
https://pmc.ncbi.nlm.nih.gov/articles/PMC8378355/ -
DrugBank detailed entry for SNAC and related compounds:
https://go.drugbank.com/drugs/DB16693
Reviews
There are no reviews yet.