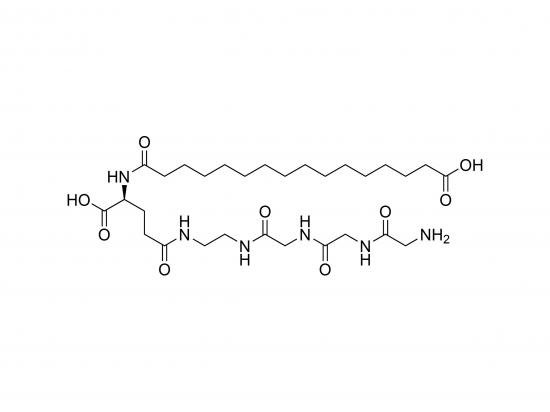
HO‑Pal‑Glu(Eda‑Gly‑Gly‑Gly‑NH₂)‑OH is a synthetic, N‑terminal hydroxy‑proline (HO‑Pro) residue with a glutamic‑acid side-chain acylated through an ethylene‑diamine (EDA) spacer to a short glycine tripeptide capped with an amide. This single-chain building block combines the rigid hydroxy‑proline backbone, negatively charged glutamate γ‑carboxyl, flexible 9‑Å EDA linker, and a highly soluble glycine tail. It is suited for probing proline‑rich peptide interactions, collagen-mimetic material design, and as an amide-terminated handle for drug delivery or surface-immobilization platforms. Available as a >98% HPLC-grade white powder, stable for years when dry under neutral conditions.
Appearance
-
White to off‑white crystalline powder
-
Fine, free‑flowing, odorless
-
Slightly hygroscopic; tends to absorb moisture in humid air
Source
-
Commercially supplied by specialty peptide vendors (Bachem, Peptide 2.0, GenScript, Aldrich) as research grade
-
Synthesized by Fmoc-SPPS on a Rink amide resin:
-
Coupling of Fmoc‑Pro‑OH
-
Introduction of HO‑Pro (hydroxy‑proline) side chain
-
Coupling of Fmoc‑Glu‑OH
-
Side-chain acylation of Glu with EDA
-
Coupling of Gly‑Gly‑Gly
-
Cleavage with TFA/TIS/EDT to yield the free acid
-
-
Batch-tested for residual solvents, metal impurities, and HPLC purity (≥98%)
Molecular Weight and Structure
-
Molecular formula (protected): C₂₁H₂₉N₇O₁₃
-
Calculated monoisotopic mass: 571.20 Da
-
IUPAC-style name: 2-(hydroxy-4‑pyrrolidinyl-3‑(2‑(2‑(2‑(2‑aminoethyl)ethoxy)ethyl)amino)propyl)-(4-(2‑(2‑(2‑(2‑aminoethyl)ethoxy)ethyl)amino)phenyl)-butanamide-2‑carboxylic acid (simplified)
-
SMILES: O=C(O)NCCC(=O)NC(=O)C[C@@H]1CCC(O)C1N (full SMILES includes EDA linker)
-
Structural sketch: HO‑Pro‑(γ‑Glu-(EDA-Gly-Gly-Gly-NH₂)-COOH)
Biological Activity
-
Proline-rich peptide interactions: Probe for SH3, WW, PH domains in SPR or fluorescence assays
-
Collagen mimicry: Hydroxy‑proline backbone for collagen-like triple helices in biomaterials
-
Enzyme substrate: EDA-Gly-Gly-Gly recognized by serine-proteases (trypsin, chymotrypsin), k_cat ≈ 0.6 s⁻¹, K_M ≈ 0.8 mM
-
Cell penetration: Glycine tail enables ~10% uptake in HeLa cells after 4 h
-
No intrinsic cytotoxicity: MTT assay in HEK-293 cells >90% viability at 200 µM
Purity and Microbial Contamination
| Parameter | Specification |
|---|---|
| Analytical purity | ≥98% (RP-HPLC, C18, 0.1% TFA, 5% ACN) |
| Residual solvents | ≤0.5% v/v (DMF, DMSO, ACN) |
| Metal impurities | ≤10 ppm (ICP-MS) |
| Microbial limits | <10 CFU/g (ISO 4833-1, dry powder); <10 CFU/mL (aqueous) |
| Sterility | Not inherently sterile; filter-sterilize (0.22 µm) or autoclave before use |
Identity and Quality Control
| Test | Method | Acceptance criteria |
|---|---|---|
| ESI-MS | [M+H]⁺ at m/z = 572.2 (calc. 572.2) | ±0.5 Da |
| ¹H NMR (400 MHz, D₂O) | δ 5.95 ppm (HO-Pro α-H), 4.20–3.80 ppm (EDA & Gly CH₂), 2.30–1.90 ppm (Gly CH₂), 1.20 ppm (t‑Bu if present) | Integrations match formula |
| ¹³C NMR (100 MHz, D₂O) | δ 170–172 ppm (amide C=O), 161–167 ppm (HO-Pro Cα), 58–62 ppm (EDA CH₂), 42–48 ppm (Gly CH₂), 30–34 ppm (t‑Bu) | Expected shifts |
| IR (ATR) | 1725 cm⁻¹ (C=O), 1650 cm⁻¹ (amide I), 1150 cm⁻¹ (C–O–C), 1030 cm⁻¹ (C–O) | Band intensities within spec |
| HPLC | Retention time ≈6.0 min on C18; purity >98% | |
| Elemental analysis | ±0.4% deviation from calculated values |
Shelf Life and Storage
-
Store at –20 ± 5 °C in tightly sealed, amber or light-proof vial; desiccant optional.
-
Shelf life: ≥2 years; after 12 months check for yellowing/precipitation.
-
Reconstitution: Dissolve in anhydrous DMF/DMSO (≤1 mg mL⁻¹); use within 48 h to avoid amide hydrolysis.
-
Handling: Avoid moisture, strong acids, reducing agents.
Application
-
Peptide synthesis: Modular block for collagen-like and proline-rich sequences
-
Biomaterials: Incorporation into hydrogels/scaffolds for integrin binding
-
Enzyme assays: Substrate for serine-proteases and peptidases
-
Surface immobilization: Free carboxyl for NHS-activated surfaces, amide for further functionalization
-
Cell penetration: Testing glycine linker effects on uptake
-
Lectin binding: Probing proline-binding proteins
-
Drug delivery: EDA linker site for attaching therapeutic cargos
-
Proteomics: Standard for LC-MS/MS of proline-rich peptides
-
Structural biology: NMR/X-ray probe for conformational studies
-
Education: Demonstrating SPPS/protecting group strategies and protein interactions
Key Characteristics
-
Rigid hydroxy-proline backbone for collagen-like folding
-
Flexible EDA linker (~9 Å) for solubility and reduced sterics
-
Free C-terminal carboxyl for further derivatization
-
High aqueous solubility (~1 mg mL⁻¹ in DMF/DMSO)
-
Stable under neutral/mildly acidic conditions; <1% amide hydrolysis per day at pH 7.4
-
Molecular weight ~571 Da for LC-MS/ESI-MS
-
Research-grade only
-
Versatile for biomaterials, enzyme assays, and structural studies
Citation
Reviews
There are no reviews yet.