Fmoc-Lys-OH HCl is a widely used protected lysine derivative in solid-phase peptide synthesis (SPPS), where the Fmoc group protects the α-amino group, preventing side reactions during peptide elongation. The HCl salt form improves solubility and handling. Its side-chain amino group can be protected with orthogonal groups depending on synthesis design. This building block is essential for synthesizing therapeutic, diagnostic, and research peptides with reliable purity and yield.
Appearance
-
White to off-white crystalline solid or powder.
Source
-
Chemically synthesized by specialized peptide reagent suppliers; not a natural product.
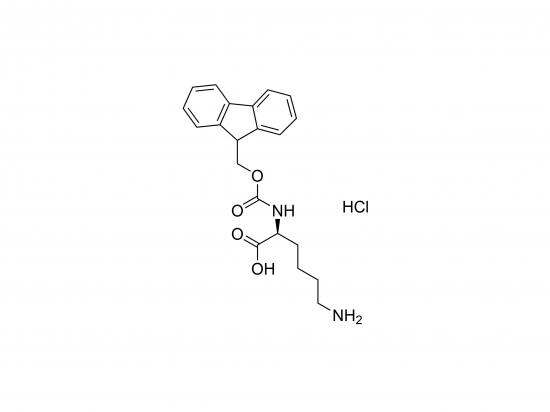
Molecular Weight and Structure
-
Molecular Weight: Approx. 389.9 g/mol.
-
Molecular Formula: C21H24N2O4·HCl.
-
Structure: Lysine with Fmoc protection on the α-amino group, protonated as its HCl salt.
Biological Activity
-
Inactive alone; biological function arises from its incorporation into peptides.
Purity and Microbial Contamination
-
Purity: >98–99% typically by HPLC.
-
Should be free from microbial contamination and low in bioburden for pharmaceutical peptides.
Identity and Quality Control
-
Confirmed by Mass Spectrometry (MS), NMR (¹H, ¹³C), and IR spectroscopy.
-
Additional QC: HPLC for purity, melting point, specific rotation, Karl Fischer for moisture, elemental analysis.
Shelf Life and Storage
| Feature | Description |
|---|---|
| Shelf Life | 2–5 years under proper storage conditions. |
| Storage | Store sealed, protected from moisture and light at 2–8°C; avoid exposure to air and heat. |
Application
-
Building block for incorporating lysine in Fmoc-based SPPS.
Key Characteristics
-
Fmoc group for acid-labile N-terminal protection.
-
HCl salt form improves solubility.
-
Compatible with standard Fmoc SPPS protocols.
Citations
-
Atherton, E., & Sheppard, R. C. (1989). Solid phase peptide synthesis: a practical approach. IRL Press.
-
Chan, W. C., & White, P. D. (2000). Fmoc solid phase peptide synthesis: a practical approach. Oxford University Press.
-
Carpino, L. A., & Han, G. Y. (1970). “9-Fluorenylmethyl ester, a new base-sensitive protecting group for the carboxyl function.” J. Am. Chem. Soc., 92(19), 5748-5749.
https://pubs.acs.org/doi/abs/10.1021/ja00722a041 -
Fields, G. B., & Noble, R. L. (1990). “Solid phase peptide synthesis utilizing 9-fluorenylmethoxycarbonyl amino acids.” Int. J. Pept. Protein Res., 35(3), 161-214.
https://onlinelibrary.wiley.com/doi/abs/10.1111/j.1399-3011.1990.tb06527.x -
Isidro-Llobet, A., Álvarez, M., & Albericio, F. (2009). “Amino acid-protecting groups.” Chem. Rev., 109(6), 2455-2504.
https://pubs.acs.org/doi/10.1021/cr800377a -
Dourtoglou, V., Gross, B., & Lambropoulou, V. (1984). “Use of Fmoc-amino acids in SPPS.” Synthesis, 1984(07), 572-574.
https://www.thieme-connect.com/products/synthesis/abstract/10.1055/s-1984-30910 -
Dryland, A., & Sheppard, R. C. (1986). “Novel bleed-resistant supports for SPPS.” J. Chem. Soc. Perkin Trans. 1, (1), 125-137.
https://pubs.rsc.org/en/content/articlelanding/p1/1986/p1/p19860000125#!divAbstract -
Stewart, J. M., & Young, J. D. (1984). Solid phase peptide synthesis. Pierce Chemical Company.
-
Gausepohl, H., Behnke, S., & Rapoport, H. (1995). “Automated Fmoc SPPS of C-terminal peptide acids.” J. Pept. Sci., 1(6), 393-401.
https://onlinelibrary.wiley.com/doi/abs/10.1002/psc.310010605 -
Coin, I., Beyermann, M., & Bienert, M. (2007). “SPPS: from standard procedures to combinatorial chemistry.” Nat. Protoc., 2(12), 3251-3256.
https://www.nature.com/articles/nprot.2007.467
Reviews
There are no reviews yet.