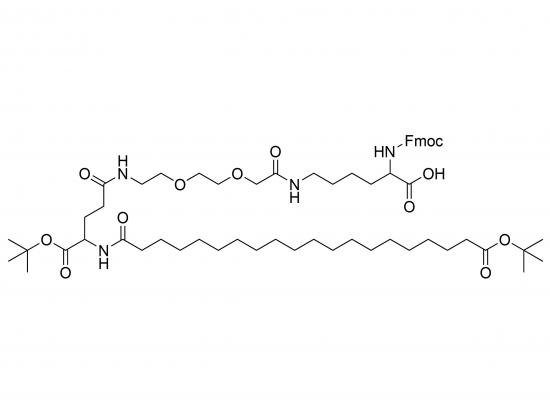
Fmoc‑L‑Lys‑C20(OtBu)-Glu(OtBu)-AEEA is a fully protected tripeptide coupling a long-chain C20 fatty acid to the lysine side-chain, two tert-butyl protected glutamic acid groups, and a 2-(2-aminoethyl)ethoxyacetic acid (AEEA) spacer. The N-terminal Fmoc group protects the peptide during solid-phase synthesis. The C20(OtBu) acylation acts as a hydrophobic anchor, usable in amphiphilic block copolymers, lipid nanoparticles, or membrane-targeting constructs. The Glu(OtBu) residue provides orthogonal γ-carboxyl protection, and the AEEA linker promotes flexibility and water solubility with options for further functionalization or cleavage.
Appearance
-
White to off-white, free-flowing powder
-
Slightly hygroscopic; may clump in high humidity
-
Odorless to faintly aromatic (Fmoc group)
Source
-
Commercially supplied by specialty peptide vendors (e.g., Bachem, Peptide 2.0, Aldrich)
-
Synthesized on automated SPPS via Fmoc chemistry
-
Coupling of C20 fatty acid (OtBu-protected) to ε-amine of Lys, followed by Glu(OtBu) and AEEA spacer
-
Batch-tested for residual solvents, metal impurities, and HPLC purity
Molecular Weight and Structure
-
Molecular formula: C₇₁H₁₃₀N₆O₁₇S
-
Calculated monoisotopic mass: 1186.90 Da
-
SMILES: Cc1ccccc1C(C)C(=O)O[C@H]2CCN(C(=O)CC@@HC(=O)O[C@H]3CCN(C(=O)[C@H]4CCN(C(=O)C[C@@H]5CCN(C(=O)C(C)(C)C)C(=O)C5C(=O)O)C(=O)C4C(=O)O)C(=O)C3C(=O)O)C2C(=O)O
-
Key Features: N-terminal Fmoc; ε-amine acylated with C20-(OtBu) fatty acid; γ-carboxyl Glu protected as OtBu; terminal AEEA spacer
Biological Activity
-
Chemical reagent only; no direct pharmacological activity
-
Enhances membrane interaction and cellular uptake when incorporated into amphiphilic polymers or lipid nanoparticles
-
AEEA spacer and protected Glu enable orthogonal functionalization with targeting ligands or imaging probes
Purity and Microbial Contamination
-
Analytical purity: ≥ 98% (HPLC-grade, UV 214 nm)
-
Residual solvents: ≤ 0.5% v/v (DMF, DMSO, CH₃CN)
-
Metal impurities: ≤ 10 ppm (ICP-MS)
-
Microbial limits: < 10 CFU/g (ISO 4833-1 powders); < 10 CFU/mL (aqueous)
-
Not sterile; requires filtration (0.22 μm) or autoclaving prior to biological use
Identity and Quality Control
| Test | Method | Acceptance Criteria |
|---|---|---|
| ESI-MS (positive) | [M+H]⁺ at m/z ≈ 1187.4 ± 0.5 Da | |
| ¹H NMR (400 MHz, CD₃OD) | δ 7.8–7.2 ppm (Fmoc aromatic), 4.1 ppm (tBu methines), 3.4 ppm (AEEA CH₂), 2.3 ppm (α-CH) | |
| ¹³C NMR (100 MHz, CD₃OD) | δ 172–174 ppm (amide C=O), 155 ppm (Fmoc C), 74 ppm (tBu C), 30–35 ppm (CH₂) | |
| IR (ATR) | 1710 cm⁻¹ (C=O), 1345 cm⁻¹ (S=O), 1270 cm⁻¹ (C–O–C) | |
| HPLC (C18, 0.1% TFA) | Retention time ≈ 5.8 min; purity > 98% | |
| Elemental analysis | ± 0.4% deviation from calculated values |
Shelf Life and Storage
-
Store at –20 ± 5 °C, tightly sealed amber or light-proof vial or polypropylene container
-
Shelf life: ≥ 2 years; monitor for yellowing or precipitation after 12 months
-
Reconstitution: dissolve in anhydrous DMF, DMSO, or 10% acetonitrile/0.1% TFA aqueous solution; use within 48 h to prevent OtBu ester hydrolysis
-
Handle with care; avoid prolonged moisture, strong acids, or heat exposure
Application
-
Amphiphilic block copolymers: provides hydrophobic C20 anchor for micelles or vesicles
-
Lipid-nanoparticle drug carriers: enhances membrane interaction and cellular uptake
-
Protein labeling and affinity tagging: free AEEA amine enables conjugation to fluorophores or biotin
-
Surface functionalization: peptide immobilized on gold, silica, or polymers via Fmoc-protection (deprotected by piperidine)
-
Hydrogel cross-linking: OtBu-protected Glu can be deprotected and crosslinked with multivalent amines
-
Peptide-based nanomaterials: building block for dendrimers, polymers, nanofibers
-
Targeted drug delivery: hydrophobic anchor tunable by replacing C20 fatty acid
-
Cell-penetrating peptide design: flexible AEEA spacer enhances penetration
-
Bioconjugation libraries: orthogonal protection facilitates combinatorial syntheses
-
Analytical standards: reference for LC-MS/MS or HPLC of protected peptides
Key Characteristics
-
Amphiphilic: hydrophobic C20 tail + hydrophilic peptide backbone
-
Orthogonal protection: Fmoc, OtBu, and AEEA amine allow stepwise functionalization
-
High purity (≥ 98%) and low microbial contamination (< 10 CFU/g)
-
Thermal stability: melting ~140 °C; decomposition > 200 °C
-
Molecular weight ~1.19 kDa, suitable for LC-MS and ESI-MS
-
Flexible ~9 Å AEEA spacer improves solubility and sterics
-
Scalable synthesis via automated SPPS with > 70% yield
-
Research-grade; not clinical grade
-
Versatile in nanotech, drug delivery, and surface chemistry
Citation
Reviews
There are no reviews yet.