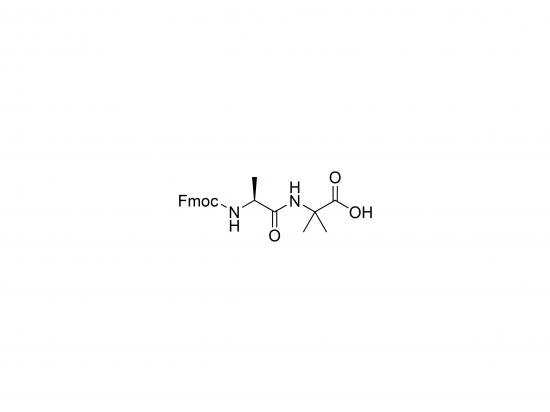
Fmoc-Ala-Aib-OH is a dipeptide building block primarily employed in solid-phase peptide synthesis (SPPS) for constructing peptides with defined structural properties. It combines alanine (Ala), a small, hydrophobic proteinogenic amino acid, with α-aminoisobutyric acid (Aib), a non-proteinogenic amino acid noted for its helix-inducing effect. The N-terminal Fmoc (9-fluorenylmethoxycarbonyl) protective group facilitates controlled SPPS. Aib introduces conformational constraint, stabilizing α-helical and 3₁₀-helical structures, enhancing peptide stability and resistance to enzymatic degradation. This versatility is beneficial in drug design, biomaterial engineering, and studies of peptide folding and function.
Appearance
- White to off-white solid powder
Source
- Synthetic chemical production
- Commercial availability from peptide synthesis reagent suppliers
Molecular Weight and Structure
- Approx. 396.4 g/mol (C₂₁H₂₂N₂O₅; may vary with salt/hydrate forms)
- Contains:
- Fmoc group on N-terminus
- Alanine (Ala) residue
- α-Aminoisobutyric acid (Aib) residue
- Free carboxyl group at C-terminus
Biological Activity
- Biologically inactive as a standalone dipeptide
- Activity is imparted when incorporated into peptide sequences
- Aib induces helical structure, Alanine offers hydrophobic interactions
Purity and Microbial Contamination
| Test | Specification |
|---|---|
| Purity | ≥ 95% (HPLC analysis) |
| Microbial contamination | Should be absent/minimal; verified by supplier COA |
| Endotoxin/sterility tests | Bacterial endotoxin test (LAL), sterility testing where relevant |
Identity and Quality Control
| Test | Specification |
|---|---|
| Mass Spectrometry | Confirms molecular weight |
| NMR Spectroscopy | ¹H/¹³C NMR confirms chemical structure |
| IR Spectroscopy | Identifies characteristic functional groups |
| HPLC | Determines purity/impurities |
| Optical Rotation | To confirm L-Alanine stereochemistry |
| Amino Acid Analysis | Confirms correct amino acid composition after hydrolysis |
Shelf Life and Storage
| Feature | Description |
|---|---|
| Shelf Life | Typically 1–2 years from manufacture (confirm with supplier COA) |
| Storage | Store at –20°C or below, inert atmosphere (argon/nitrogen), tightly sealed container, protected from moisture/light; avoid repeated freeze-thaw cycles |
Application
- Building block for SPPS in peptide chain construction
- Synthesis of α-helical and 3₁₀-helical peptides
- Peptidomimetic development
- Drug discovery for peptides targeting protein-protein interactions
- Engineering biomaterials with tailored structural properties
- Exploration of peptide folding and structure-function relationships
Key Characteristics
- N-terminal Fmoc protection (base-labile deprotection)
- Aib inclusion for helix stabilization and resistance to cleavage
- Alanine contributes hydrophobicity and molecular compactness
- Soluble in DMF, DMSO, acetonitrile
- Versatile for coupling with other amino acids
Citation
- Search for “Fmoc-Ala-Aib-OH peptide synthesis” for direct usage in SPPS
- Explore “Aib peptide helix” or “α-aminoisobutyric acid peptide conformation” for conformational studies
- Look up “helical peptide design” and “310-helical peptide synthesis” for design methodologies
- Search “peptidomimetics” and “drug discovery” with mention of Aib for biomedical applications
- Review technical data sheets from suppliers
- Use Reaxys, SciFinder, PubMed, Google Scholar with terms like “Fmoc-Ala-Aib-OH synthesis”, “Aib peptide helix”, “Aib conformation”, or “Aib-containing peptides” for relevant publications
Reviews
There are no reviews yet.