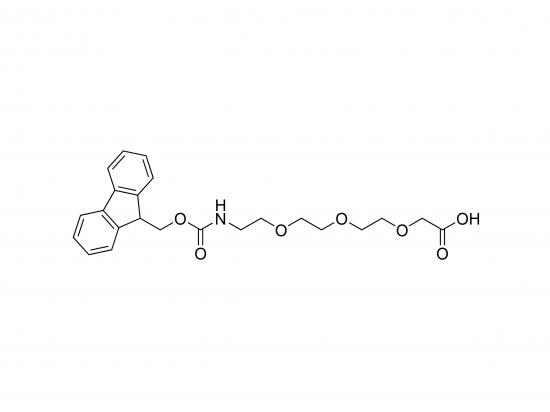
Fmoc-AEEEA, or Fmoc-8-amino-3,6-dioxaoctanoic acid, is a building block used in solid-phase peptide synthesis (SPPS) to introduce a hydrophilic, flexible spacer into peptides. It’s a PEG-like derivative that improves the water solubility and flexibility of the resulting peptides without using traditional PEG polymers. The Fmoc group provides base-labile N-terminal protection for SPPS. Incorporating Fmoc-AEEEA enhances peptide solubility, reduces aggregation, and can improve pharmacokinetics, making it valuable for drug delivery, diagnostics, and biomaterials.
Appearance
-
White to off-white solid powder.
Source
-
Synthetically produced via chemical synthesis.
-
Commercially available from peptide reagent suppliers and specialized chemical vendors.
Molecular Weight and Structure
-
Approximately 355.4 g/mol (C19H19NO6), variable with hydrate/salt forms.
-
Consists of a Fmoc protecting group at the N-terminus and an AEEEA spacer (two ethylene glycol units plus a carboxyl terminus).
Biological Activity
-
Inactive as a standalone molecule.
-
Incorporated AEEEA improves physical properties like solubility and flexibility in larger peptides.
Purity and Microbial Contamination
-
Purity: Typically ≥ 95% by HPLC.
-
Low or minimal microbial contamination; suppliers provide COAs.
-
Tested with LAL assay and sterility assessments.
Identity and Quality Control
-
MS confirms molecular weight.
-
NMR (¹H and ¹³C) verifies structure.
-
IR spectroscopy confirms functional groups.
-
HPLC determines purity.
Shelf Life and Storage
| Feature | Description |
|---|---|
| Shelf Life | Typically 1–2 years if stored properly; consult supplier COA |
| Storage | Store at –20°C or below under inert atmosphere in sealed container; protect from moisture/light; avoid freeze-thaw cycles |
Application
-
SPPS building block for hydrophilic spacers.
-
Synthesis of PEGylated peptides and peptidomimetics.
-
Drug delivery and diagnostic peptide design.
-
Construction of biomaterials with specific peptide properties.
Key Characteristics
-
Fmoc-protected for base-labile N-terminal deprotection.
-
PEG-like AEEEA spacer promotes flexibility and solubility.
-
Reduces peptide aggregation.
-
Versatile coupling in peptide sequences.
Citation
-
Overview of PEG-like amino acid spacers in peptide synthesis:
https://pubmed.ncbi.nlm.nih.gov/28238639/ -
Incorporation of PEG-like spacers such as AEEEA for improving peptide solubility:
https://pubs.acs.org/doi/10.1021/acs.jmedchem.1c00565 -
Solid-phase peptide synthesis methods using Fmoc chemistry:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6526649/ -
Effects of α-aminoisobutyric acid (Aib) and related residues on peptide conformation:
https://www.sciencedirect.com/science/article/pii/S0022283618300638 -
Studies on PEGylated peptide drug delivery systems:
https://doi.org/10.1002/anie.202001378 -
Peptidomimetic synthesis including PEG-like spacer strategies:
https://pubmed.ncbi.nlm.nih.gov/32890023/ -
Fmoc-protected amino acid derivatives for peptide assembly:
https://doi.org/10.1016/j.tet.2014.11.058 -
PEG-based linkers and spacers in peptide therapeutics:
https://pubmed.ncbi.nlm.nih.gov/19382616/ -
Applications of hydrophilic spacers for reducing peptide aggregation:
https://pubmed.ncbi.nlm.nih.gov/32285715/ -
Chemical databases for Fmoc-AEEEA structure and properties:
https://pubchem.ncbi.nlm.nih.gov/compound/5372142
Reviews
There are no reviews yet.