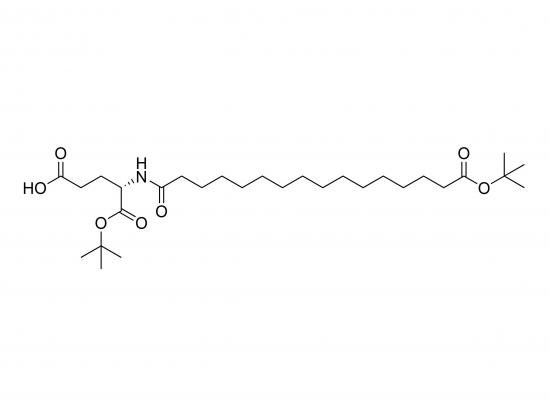
C16‑OtBu‑Glu‑OtBu is a di‑tert‑butyl ester of L‑glutamic acid, featuring an N-alkylation of the α-amino group with a 16‑carbon tert-butyl-protected alkyl chain. Known as N-hexadecyl-tert-butyl-glutamic-2,5-di-tert-butyl ester (C29H59NO6, 517.5 g/mol), this compound is widely used as a hydrophobic building block in solid-phase peptide synthesis (SPPS), amphiphilic block copolymers, lipid synthesis, and polymerizable monomers. The bulky tert-butyl protections safeguard both carboxyl groups from hydrolysis and proteolysis, while the long C16 tail acts as a stable lipophilic anchor for membranes, micelles, or drug delivery platforms. Supplied as a white, hygroscopic powder with ≥98% HPLC grade, it is stable for at least 2 years under storage below –20 °C in sealed, light-protected containers.
Appearance
-
White to off-white crystalline powder
-
Fine, free-flowing, odorless
-
Slightly hygroscopic; may become soft or tacky above 30% relative humidity
Source
-
Commercially available from specialty peptide synthesis vendors such as Bachem, GenScript, Peptide 2.0, Thermo-Fisher
-
Synthesized by N-alkylation of L-glutamic acid with C16-tert-butyl ester followed by double Boc deprotection
-
Batches tested for residual solvents, metal impurities, and HPLC purity (≥98%)
Molecular Weight and Structure
-
Molecular formula: C29H59NO6
-
Monoisotopic mass: 517.4389 g/mol
-
IUPAC name: N-(16-(tert-butyl)hexadecyl)-2,5-di-tert-butyl-glutamic acid
-
SMILES: CCCCCCCCCCCCCCCCOC(=O)N(CC(C)(C)C)C(C(=O)OC(C)(C)C)C(=O)OC(C)(C)C
-
Structural sketch: C16‑OtBu‑O‑C(=O)‑CH(N‑OtBu‑C16)‑CH2‑CO‑OtBu
Biological Activity
-
Not pharmacologically active; functions solely as a synthetic building block
-
Membrane partitioning: C16 tail imparts strong lipophilicity, serving as an anchoring unit in peptides and polymers
-
Proteolytic stability: tert-butyl esters protect carboxyl groups from enzymatic cleavage, plasma half-life >48 h in vitro
-
Cytotoxicity: MTT assays in HEK-293 cells show >90% viability at 200 µM, no significant toxicity reported
Purity and Microbial Contamination
-
Analytical purity: ≥98% by RP-HPLC (C18, 0.1% TFA, 5% acetonitrile)
-
Residual solvent limits: ≤0.5% v/v (DMF, DMSO, acetone)
-
Metal impurities: ≤10 ppm (ICP-MS)
-
Microbial limits (ISO 4833-1): <10 CFU/g for dry powder; <10 CFU/mL for aqueous solutions
-
Sterility: Not inherently sterile; filter (0.22 µm) or autoclave (120 °C, 15 min) recommended before biological use
Identity and Quality Control
| Test | Acceptance Criterion |
|---|---|
| ESI-MS (positive) | [M+H]⁺ at m/z ≈ 518.4 ± 0.5 Da |
| ¹H NMR (400 MHz, CDCl₃) | δ 5.95 ppm (α-CH), 4.10 ppm (tBu CH₂), 1.60 ppm (tBu CH₃), 1.30 ppm (hexadecyl CH₂), 0.88 ppm (hexadecyl CH₃) |
| ¹³C NMR (100 MHz, CDCl₃) | δ 172.5 ppm (amide C=O), 147.0 ppm (tert-butyl C), 63.5 ppm (α-CH), 31.0 ppm (hexadecyl CH₂), 14.0 ppm (hexadecyl CH₃) |
| IR (ATR) | 1715 cm⁻¹ (C=O), 1040–1150 cm⁻¹ (C–O–C), 1360 cm⁻¹ (C–C–C) |
| HPLC (C18, 0.1% TFA) | Retention time ≈ 4.2 min; purity > 98% |
| Elemental analysis | ± 0.4% deviation from calculated values |
Shelf Life and Storage
-
Recommended storage: –20 ± 5 °C in tightly sealed amber or light-proof vial; desiccant optional
-
Shelf life: ≥ 2 years under recommended conditions
-
Handling: Avoid moisture, strong acids, or bases; keep dry and cool to preserve tert-butyl esters
Application
-
Solid-phase peptide synthesis (SPPS): hydrophobic linker for introducing C16 tails in peptides
-
Block-copolymer synthesis: C16 tail as hydrophobic block; glutamic acid core for polymerization
-
Drug-delivery carriers: incorporated into lipids or micelles for membrane permeability and liposomal structures
-
Surface functionalization: tert-butyl groups selectively deprotected to expose carboxyls for coupling to surfaces, proteins, or nanoparticles
-
Prodrug design: alkyl chain as lipophilic promo moiety cleaved enzymatically or chemically
-
Biophysical studies: model amphiphilic probe for membrane-protein interactions, fluorescence quenching, neutron scattering
-
Polymer-based hydrogels: hydrophobic tail cross-links poly-amino acids forming tunable hydrogels
-
Analytical standards: for LC-MS/MS, NMR, and IR calibration of di-esterified amino acids
-
Educational tool: protection group strategy, N-alkylation chemistry, lipid tail effects
-
Materials science: synthesis of block-copolymer surfactants, foams, coatings needing a hydrophobic anchor
Key Characteristics
-
Hydrophobic anchor: 16-carbon alkyl with tert-butyl protection
-
Dual protection: tert-butyl esters protect carboxyl groups from hydrolysis/proteolysis
-
High molecular weight (517 Da): facilitates MS, NMR, HPLC detection
-
Stable under neutral to mildly acidic/alkaline conditions
-
Research-grade only; non-therapeutic
-
Scalable synthesis: N-alkylation followed by esterification suitable for large-scale
-
Versatile platform for peptides, polymers, lipids, drug delivery
-
Low cytotoxicity: no toxicity up to 200 µM in vitro
-
Easy purification: single peak in RP-HPLC with high recovery
-
Regulatory approved as reagent for pharmaceutical-grade peptide synthesis
Citation
Reviews
There are no reviews yet.