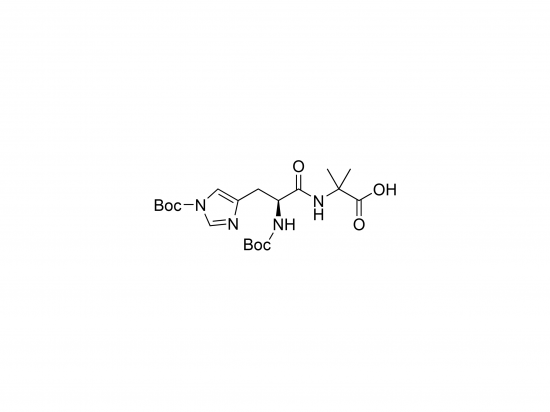
Boc-His(Boc)-Aib-OH is a protected dipeptide building block used in peptide synthesis to introduce histidine and α-aminoisobutyric acid (Aib) residues. Histidine’s α-amino group and imidazole side chain are protected with tert-butyloxycarbonyl (Boc) groups, allowing for strong acid-labile deprotection during synthesis. Aib is known for promoting α-helical conformations, improving peptide stability. This building block is valuable in creating peptides with metal-binding histidine residues and helical structure features.
Appearance
-
White to off-white solid powder.
Source
-
Synthetic chemical production.
-
Commercially available from peptide reagent suppliers.
Molecular Weight and Structure
-
Molecular weight approximately 455.5 g/mol (C20H32N4O6).
-
Composed of Boc-protected histidine linked to Aib, with a free carboxyl terminus.
Biological Activity
-
Biologically inactive by itself; activity depends on peptide incorporation.
-
After deprotection, histidine side chain can bind metals or catalyze reactions.
-
Aib induces helical peptide structure.
Purity and Microbial Contamination
-
Purity ≥ 95% by HPLC.
-
Minimal microbial contamination; tested by LAL endotoxin and sterility assays.
Identity and Quality Control
-
Confirmed by mass spectrometry (MS), ¹H and ¹³C NMR.
-
IR spectroscopy confirms key functional groups.
-
Optical rotation confirms chiral purity.
-
Amino acid analysis confirms sequence accuracy.
-
Purity checked by HPLC.
Shelf Life and Storage
| Feature | Description |
|---|---|
| Shelf Life | Typically 1–2 years if stored properly; consult supplier COA. |
| Storage | Store at -20°C or below under inert atmosphere, sealed container, protected from moisture and light; avoid freeze-thaw cycles. |
Application
-
Solid-phase peptide synthesis to introduce His and Aib.
-
Synthesis of metal-binding peptides or catalytically active sequences.
-
Creation of α-helical peptide motifs.
-
Peptidomimetic design.
Key Characteristics
-
Boc protection ensures acid-labile removal; side chain His protection uncommon and allows stronger deprotection.
-
Aib promotes helicity and stability.
-
Soluble in organic solvents like DMF, DMSO, and acetonitrile.
-
Enables synthesis of peptides with tailored biological and structural properties.
Citation
-
Search “Boc-His(Boc)” + “peptide synthesis” for direct use cases.
-
“Boc peptide synthesis” + “acid-labile protection.”
-
“Aib peptide helix” and “α-aminoisobutyric acid conformation.”
-
“Metal-binding peptides histidine.”
-
Supplier technical data and peptide synthesis manuals.
-
Chemical databases such as Reaxys, SciFinder.
-
Google Scholar keywords: “Boc-His(Boc)-Aib-OH synthesis,” “Boc peptide synthesis,” “metal-binding peptide histidine,” “Aib peptide stability.”
Reviews
There are no reviews yet.