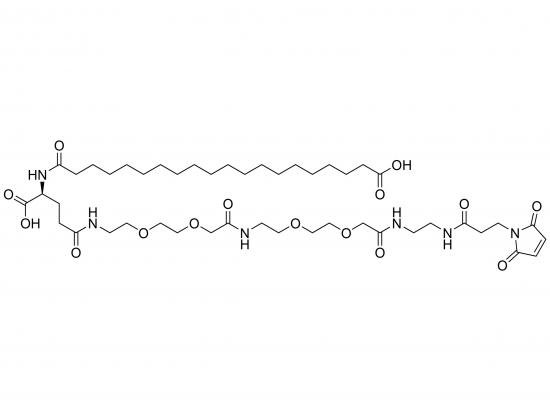
HO-Ara-Glu(AEEA-AEEA-Mal)-OH is a synthetic heterobifunctional crosslinker based on a glutamic acid scaffold. It incorporates arabinose (Ara) at the α-carboxyl position, linked via ester or amide bond, and a maleimide (Mal) group attached to the γ-carboxyl group via two AEEA (2-[2-(2-aminoethoxy)ethoxy]acetic acid) units as a flexible, hydrophilic spacer. The arabinose moiety provides a carbohydrate handle for lectin binding, while the maleimide selectively reacts with thiols in cysteine residues, enabling stable conjugation. The glutamic acid core allows further modification and serves as a central branching point, ideal for targeted drug delivery, protein modification, nanoparticle functionalization, and advanced biomaterials.
Appearance
-
White to off-white solid or viscous liquid
-
Likely hygroscopic
Source
-
Chemically synthesized by specialized bioconjugation reagent manufacturers or custom synthesis labs
-
Not commonly available from general suppliers
Molecular Weight
-
Approximately 607.6 g/mol (theoretical value; may vary with counterions, protecting groups, or linkage chemistry)
Structure
-
Glutamic acid core with arabinose at α-carboxyl
-
γ-Carboxyl functionalized with amide-linked AEEA-AEEA chain terminated by maleimide
Biological Activity
-
No intrinsic activity; function depends on conjugated molecules
-
PEG-like AEEA linkers improve solubility and reduce nonspecific binding
-
Arabinose may bind lectins or carbohydrate-recognizing proteins
-
Glutamic acid scaffold may affect cell interactions
Purity and Microbial Contamination
| Purity and Microbial Contamination | Specification |
|---|---|
| Purity | >90%, ideally >95% by HPLC |
| Microbial contamination | Concern for in vivo use, minimized |
| Endotoxin levels | Should be controlled |
| Certificate of Analysis (CoA) | Includes purity, residual solvents, endotoxin |
Identity and Quality Control
| Identity and Quality Control | Specification |
|---|---|
| Mass Spectrometry (MS) | Confirms molecular weight |
| Nuclear Magnetic Resonance (¹H & ¹³C NMR) | Confirms chemical structure |
| High-Performance Liquid Chromatography (HPLC) | Assesses purity and impurities |
| Functional assays | Verify arabinose and maleimide reactivity |
| Confirmation of free carboxylic acids | Verified analytically |
Shelf Life and Storage
-
Store at –20°C, dry, inert atmosphere (argon or nitrogen)
-
Protect from light and moisture
-
Maleimide group sensitive to hydrolysis; avoid water exposure
-
Shelf life ~6–12 months
Applications
-
Protein-carbohydrate conjugation via arabinose and maleimide
-
Surface modification with carbohydrate handles
-
Antibody-drug conjugates (ADCs) incorporating hydrophilic spacers
-
Nanoparticle functionalization for targeted delivery
-
Crosslinking thiol-containing entities with sugar-based ligands
Key Characteristics
-
Heterobifunctional: arabinose and maleimide reactive groups
-
Hydrophilic PEG-like AEEA spacer for reduced nonspecific binding
-
Biocompatible design
-
Maleimide selectively reacts with sulfhydryl groups
-
Carbohydrate moiety enables specific molecular recognition
-
Glutamic acid scaffold provides branching and modification points
-
Extended flexible spacer enhances bioconjugate stability and function
Citation
-
Glutamic Acid Scaffolds: Search “Glutamic acid-based dendrimers for drug delivery”
-
Maleimide Chemistry: Search “Maleimide-thiol coupling for protein conjugation”
-
Carbohydrate-Protein Conjugates: Search “Carbohydrate-modified proteins for targeted drug delivery”
-
AEEA Linkers: Search “AEEA linkers improve solubility and reduce aggregation” (Bioconjugate Chemistry)
-
Maleimide-Thiol Drug Delivery: Search “Maleimide-thiol coupling for controlled drug release”
-
Arabinose Targeting: Search “Arabinose-modified nanoparticles for targeted drug delivery”
-
PEGylation for Biocompatibility: Search “PEGylation to reduce immunogenicity of proteins”
-
ADC Linker Design: Search “Optimizing linker design for antibody-drug conjugates”
-
Multivalent Carbohydrate Display: Search “Multivalent carbohydrate display on protein scaffolds”
-
Amino Acid Linkers: Search “Amino acids as building blocks for bioconjugation”
-
Flexible Spacers: Search “Flexible linkers enhance bioactivity of conjugated molecules”
Reviews
There are no reviews yet.