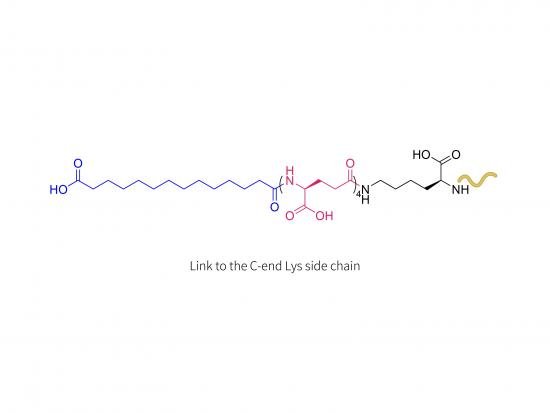
Insulin 0143-0406 (LYS) is an investigational long-acting basal insulin analogue under clinical development for type 2 diabetes treatment. This molecule is a modified human insulin with a lipophilic moiety, likely a long-chain fatty acid, conjugated at a specific lysine (LYS) residue. This acylation enhances binding to albumin in the bloodstream, considerably extending the half-life and enabling less frequent dosing compared to standard basal insulins.
Appearance:
-
Presumed clear, colorless solution for subcutaneous injection, similar to other insulin analogues.
Source:
-
Produced via recombinant DNA technology in expression systems such as Saccharomyces cerevisiae or E. coli.
-
The insulin is chemically modified post-expression with the lipophilic moiety.
Molecular Weight:
-
Higher than unmodified human insulin (~5.8 kDa) due to the fatty acid attachment. Exact weight varies with modification size.
Structure:
-
Human insulin amino acid sequence modified at a lysine residue by covalent linkage to a fatty acid or similar lipophilic group on the epsilon-amino side chain, critical for its pharmacological profile.
Biological Activity:
-
Stimulates glucose uptake and inhibits hepatic glucose production.
-
Binds to insulin receptors on target tissues such as muscle, liver, and adipose tissue.
-
The lysine modification prolongs duration of insulin action, facilitating extended dosing intervals.
Purity and Microbial Contamination:
-
Purity typically ≥95% by HPLC, meeting rigorous pharmaceutical-grade standards.
-
Sterility and endotoxin levels regulated and confirmed by standard assays.
Identity and Quality Control:
-
Mass spectrometry confirms correct molecular weight and modification profile.
-
Amino acid analysis and peptide mapping verify amino acid sequence and site-specific modification.
-
HPLC and SDS-PAGE used for purity and aggregation assessments.
-
Bioassays measure receptor binding and metabolic activity.
Shelf Life and Storage
| Feature | Description |
|---|---|
| Shelf Life | Data not publicly available; consult manufacturer info |
| Storage | Likely refrigerated at 2–8°C, protected from light; do not freeze |
Applications
-
Investigational treatment for basal insulin replacement in type 2 diabetes, focusing on improved patient adherence via reduced injection frequency.
Key Characteristics
-
Human insulin analogue with site-specific acylation at lysine for albumin binding.
-
Designed as an ultra-long-acting insulin allowing less frequent dosing (possibly once weekly or less).
-
Activates endogenous insulin receptor signaling pathways.
Citation
-
Clinical trial databases (clinicaltrials.gov, PubMed) for emerging data.
-
Patent literature (Google Patents, USPTO) for structural and synthetic insights.
-
Scientific publications on fatty acid-acylated insulin analogues, albumin binding, and long-acting basal insulins.
-
Keywords for literature search include “Insulin 0143-0406,” “long-acting insulin,” “acylated insulin,” “albumin binding insulin analogue,” and “insulin receptor binding.”
Reviews
There are no reviews yet.