Insulin Icodec (LYS) is an ultra-long-acting basal insulin analogue engineered for once-weekly administration in people with type 2 diabetes. It is a modified human insulin molecule with site-specific acylation at a lysine residue. This acylation involves attaching a fatty acid or other lipophilic group that promotes strong binding to albumin in the bloodstream, thereby greatly extending the half-life and enabling sustained glucose-lowering activity.
Appearance:
-
Clear, colorless solution for subcutaneous injection.
Source:
-
Produced recombinantly in Saccharomyces cerevisiae or similar expression systems.
-
Insulin is subsequently chemically modified with a lipophilic fatty acid.
Molecular Weight:
-
Greater than unmodified human insulin (~5.8 kDa) due to the fatty acid conjugation; exact weight depends on the moiety attached.
Structure:
-
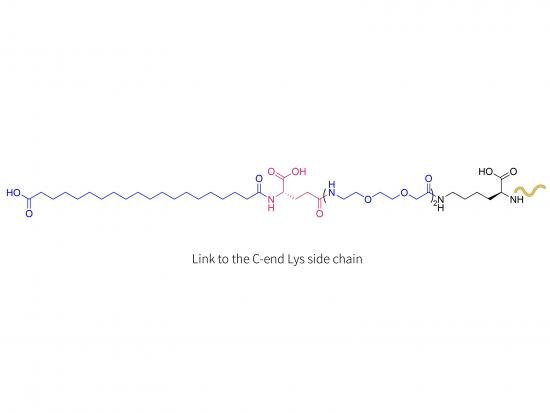
Human insulin sequence modified at a specific lysine residue by covalent attachment of a fatty acid through its epsilon-amino group.
-
This modification enhances albumin binding and alters pharmacokinetics.
Biological Activity:
-
Stimulates cellular glucose uptake and reduces hepatic glucose production.
-
Binds insulin receptors to activate signaling pathways regulating glucose homeostasis.
-
The LYS modification extends duration of action for once-weekly dosing.
Purity and Microbial Contamination:
-
Purity typically ≥95%, ensuring pharmaceutical quality.
-
Sterility and endotoxin levels meet strict regulatory thresholds.
Identity and Quality Control:
-
Confirmed by mass spectrometry for molecular weight and modification.
-
Amino acid and peptide mapping verify correct sequence and modification site.
-
HPLC and SDS-PAGE confirm purity and aggregation status.
-
Bioassays confirm activity at the insulin receptor.
Shelf Life and Storage
| Feature | Description |
|---|---|
| Shelf Life | Usually 2–3 years when stored properly; consult manufacturer’s info |
| Storage | Refrigerate at 2–8°C; protect from light; avoid freezing |
Applications:
-
Treatment of type 2 diabetes requiring basal insulin support with reduced injection frequency.
Key Characteristics:
-
Recombinant human insulin analogue.
-
Modified at lysine for ultra-long-acting properties via albumin binding.
-
Supports glucose regulation with once-weekly administration.
-
Formulated as sterile injectable solution.
Research and Information Sources:
-
FDA and EMA approval documents (status may vary; check latest updates).
-
Clinical trial databases like clinicaltrials.gov and PubMed.
-
Publications on insulin acylation and albumin-binding modifications.
-
Patent databases for structure and manufacturing details.
-
Search terms: “Insulin Icodec pharmacokinetics,” “acylated insulin,” “albumin binding insulin analogue,” “insulin receptor binding affinity.”
Reviews
There are no reviews yet.