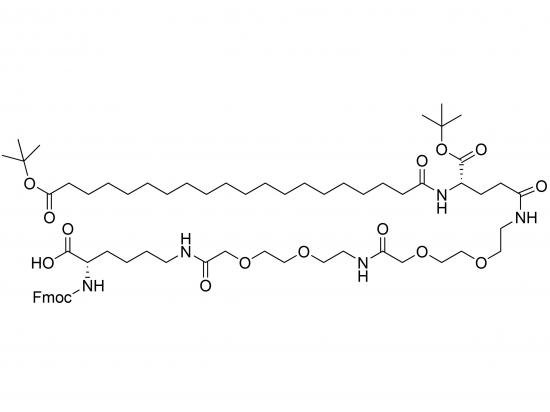
Fmoc-Lys(tBuO-Ara-Glu(AEEA-AEEA)-OtBu)-OH is a protected amino acid building block tailored for solid-phase peptide synthesis (SPPS). It features a lysine residue with the alpha-amino group protected by an Fmoc group, while the epsilon-amino group carries a side chain composed of glutamic acid linked to arabinose via a tert-butyl ester. Two AEEA units extend from the glutamic acid’s alpha-carboxyl group, with their carboxyl protected as a tert-butyl ester. The tBuO-Ara linkage may allow controlled release of arabinose, while the AEEA linkers enhance hydrophilicity and flexibility. This building block is intended for synthesizing glycopeptides with specific carbohydrate modifications for lectin targeting or glycosylation studies.
Appearance
-
White to off-white solid, typically powder or crystalline
Source
-
Chemically synthesized by specialized peptide synthesis reagent manufacturers or custom labs
-
Rarely stocked by general chemical suppliers
Molecular Weight
-
Approximately 993.1 g/mol (theoretical; may vary with counterions/protecting groups)
Structure
-
Lysine core with Fmoc on alpha-amino
-
Arabinose linked via tert-butyl ester to glutamic acid gamma-carboxyl
-
Two AEEA units attached to glutamic acid alpha-carboxyl
-
Glutamic acid carboxyl protected as tert-butyl ester
Biological Activity
-
Inherently inactive; activity depends on peptide sequence and arabinose interactions with lectins or carbohydrate-binding proteins
Purity and Microbial Contamination
| Purity and Microbial Contamination | Specification |
|---|---|
| Purity | >95% by HPLC |
| Microbial contamination | Important consideration for in vivo peptides |
| Endotoxin levels | Should be minimized |
| Certificate of Analysis (CoA) | Details purity, residual solvents, endotoxin |
Identity and Quality Control
| Identity and Quality Control | Specification |
|---|---|
| Mass Spectrometry (MS) | Confirms molecular identity |
| Nuclear Magnetic Resonance (¹H & ¹³C NMR) | Confirms structure and chemical purity |
| High-Performance Liquid Chromatography (HPLC) | Assesses purity and impurities |
| Optical Rotation | Validates stereochemistry |
| Elemental Analysis | Confirms elemental composition |
Shelf Life and Storage
-
Store at -20°C in dry, inert atmosphere (argon or nitrogen)
-
Protect from light and moisture
-
Shelf life typically 6–12 months
Application
-
Building block in SPPS for glycopeptide synthesis
-
Site-specific incorporation of arabinose carbohydrate moieties
-
Peptide design targeting lectins and carbohydrate-binding proteins
-
Studies of glycosylation patterns and carbohydrate-mediated interactions
-
Controlled release strategies via tBuO-Ara linkage
Key Characteristics
-
Fmoc protection for controlled SPPS
-
tBu protection of carboxyl groups during synthesis
-
Hydrophilic and flexible AEEA linkers increase solubility and reduce aggregation
-
Carbohydrate moiety allows targeting and biological interaction
-
Versatile for diverse peptide sequences
Citation
-
Fmoc-Lysine Derivatives: Search “Synthesis of modified peptides using Fmoc-protected lysine derivatives.”
-
Glycopeptide Synthesis: Search “Solid-phase synthesis of glycopeptides.”
-
Carbohydrate Peptides for Targeting: Search “Carbohydrate-modified peptides for targeted drug delivery.”
-
AEEA Linkers in Peptide Synthesis: Search “AEEA linkers improve peptide solubility and reduce aggregation” (Bioconjugate Chemistry).
-
t-Butyl Ester Hydrolysis: Search “Acid-labile t-butyl esters for controlled drug release.”
-
Lectin Targeting: Search “Carbohydrate-mediated targeting of liposomes to cancer cells.”
-
Solid-Phase Glycosylation: Search “Solid-phase glycosylation strategies for glycopeptide synthesis” (Chemical Reviews).
-
Fmoc-based SPPS: Search “Fmoc solid-phase peptide synthesis: A practical approach” (Methods in Molecular Biology).
-
t-Butyl Protecting Groups: Search “The use of t-butyl protecting groups in peptide synthesis” (Tetrahedron Letters).
-
Carbohydrate-Modified Amino Acids: Search “Synthesis and applications of carbohydrate-modified amino acids for SPPS” (Glycobiology).
Reviews
There are no reviews yet.