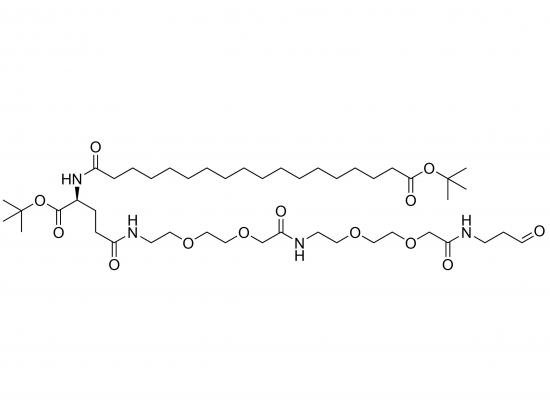
tBuO-Ste-Glu(AEEA-AEEA-NH-pALD)-OtBu is a synthetic amphiphilic molecule designed as a versatile linker or building block, most likely for use within larger structures such as liposomes, drug delivery systems, or surface modifications. The molecule combines lipophilic, hydrophilic, and reactive aldehyde functionalities. Specifically, it consists of a stearic acid (Ste) moiety esterified to glutamic acid (Glu) via a tert-butyl ester (tBuO-Ste-Glu). Two AEEA (2-[2-(2-aminoethoxy)ethoxy]acetic acid) linkers extend from the glutamic acid, terminated by an amide linked to p-ALD (para-substituted benzaldehyde). The glutamic acid’s other carboxyl group is protected as a tert-butyl ester (OtBu). The stearic acid provides lipophilicity, likely for membrane insertion or hydrophobic interactions. The AEEA linkers act as hydrophilic spacers to enhance solubility and flexibility. The p-ALD group is a reactive aldehyde moiety, which can be used for covalent conjugation to other molecules containing amine, hydrazide, or other aldehyde-reactive groups. The cleavable tBuO-Ste linkage allows for potential controlled release of stearic acid under specific conditions. The presence of the aldehyde group enables conjugation with amines or hydrazides, facilitating the formation of Schiff bases or hydrazones, respectively. This molecule is useful for creating stimuli-responsive materials, targeted drug delivery systems, or surface-modified nanoparticles.
Appearance
-
Likely a colorless to slightly yellow oil or viscous liquid, potentially a waxy solid depending on purity and storage temperature.
Source
-
Synthesized chemically, likely by specialized chemical synthesis companies or research labs specializing in linker molecules.
-
Usually not commercially available as a stock item but synthesized on demand.
Molecular Weight
-
Approximately 974.27 g/mol (Calculated based on the chemical formula; theoretical value).
Structure
-
Stearic acid linked to the γ-carboxyl of glutamic acid via a tert-butyl ester.
-
α-carboxyl of glutamic acid also protected as a tert-butyl ester.
-
Two AEEA units attached to glutamic acid via amide bonds.
-
Chain terminated with para-substituted benzaldehyde (p-ALD) also via amide bond.
Biological Activity
-
Unlikely to have inherent biological activity by itself.
-
Activity depends on what is conjugated to it.
-
Stearic acid moiety can influence membrane interactions.
-
AEEA linkers improve biocompatibility and solubility.
-
p-ALD group enables conjugation to bioactive molecules.
Purity and Microbial Contamination
| Purity and Microbial Contamination | Specification |
|---|---|
| Purity | Expected >90%, ideally >95% by HPLC |
| Microbial contamination | Less critical unless for in vivo use |
| Endotoxin levels | Should be minimized for biological applications |
| Certificate of Analysis (CoA) | Details purity, residual solvents, endotoxin if relevant |
Identity and Quality Control
| Identity and Quality Control | Specification |
|---|---|
| Mass Spectrometry (MS) | Confirms molecular weight |
| Nuclear Magnetic Resonance (¹H & ¹³C NMR) | Confirms chemical structure |
| High-Performance Liquid Chromatography (HPLC) or Thin-Layer Chromatography (TLC) | Measures purity and impurity profile |
| Aldehyde functionality verification | Confirmed by reaction with model amine & analysis by NMR or MS |
Application
-
Linker molecule to conjugate lipophilic moieties with reactive aldehydes.
-
Used in liposome modification for targeting or drug loading via surface aldehyde groups.
-
Nanoparticle surface functionalization for bioconjugation.
-
Creation of drug delivery systems with controlled release and targeting ability.
-
Surface chemistry for self-assembled monolayers or reactive coatings.
Key Characteristics
-
Lipophilic stearic acid promotes membrane insertion/hydrophobic interactions.
-
Hydrophilic AEEA linkers increase solubility and reduce steric hindrance.
-
Reactive aldehyde facilitates conjugation to amines or hydrazides.
-
Cleavable tert-butyl ester linkage allows controlled release of stearic acid.
-
Amphiphilic nature combining hydrophobic and hydrophilic properties.
-
Biocompatible, largely due to AEEA linkers.
Citation
Reviews
There are no reviews yet.