Fmoc-Pro-Aib-OH is a dipeptide building block commonly employed in solid-phase peptide synthesis (SPPS) to introduce specific structural features into peptides. It combines proline (Pro), a cyclic amino acid known for its conformational rigidity, with α-aminoisobutyric acid (Aib), a non-proteinogenic amino acid that induces helical conformations, particularly 310-helices. The Fmoc (9-fluorenylmethoxycarbonyl) group serves as the N-terminal protecting group, allowing controlled deprotection and coupling during SPPS. Together, Pro and Aib influence peptide backbone conformation, often inducing turns, bends, or helical structures. This compound is valuable in synthesizing peptides with defined secondary structures relevant in drug design, biomaterials, and protein folding studies.
Appearance
- White to off-white solid powder
Source
- Synthetically produced via chemical synthesis and commercially available from peptide synthesis reagent suppliers
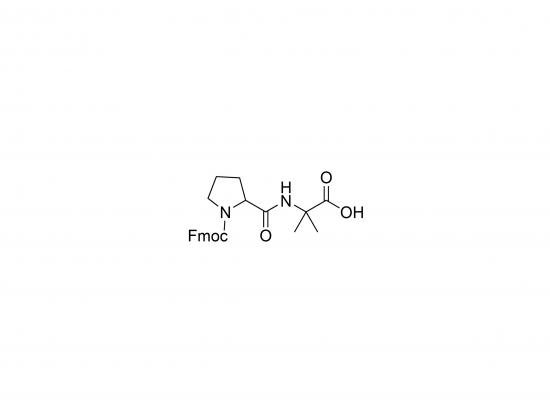
Molecular Weight and Structure
- Molecular Weight: Approximately 422.46 g/mol (based on formula C23H24N2O5)
- Structure includes:
- Fmoc protecting group at N-terminus
- Proline (Pro) residue
- α-Aminoisobutyric acid (Aib) residue
- Free carboxyl group at C-terminus
Biological Activity
- Generally biologically inactive as a standalone dipeptide
- Influences peptide conformation significantly when incorporated into larger sequences
- Proline induces kinks or turns; Aib promotes helical structures, thereby affecting peptide interactions with biological targets
Purity and Microbial Contamination
- Purity: Typically ≥ 95% confirmed by HPLC
- Microbial contamination should be absent or minimal, especially critical for biological applications
- Testing includes bacterial endotoxin assays (LAL) and sterility tests
- Suppliers provide Certificates of Analysis with microbial testing results
Identity and Quality Control
- Mass spectrometry (MS) confirms molecular weight
- NMR spectroscopy (¹H and ¹³C) verifies chemical structure
- Infrared (IR) spectroscopy confirms functional groups
- High-Performance Liquid Chromatography (HPLC) assesses purity
- Optical rotation measures enantiomeric purity (Pro residue)
- Amino acid analysis after hydrolysis confirms composition.
Shelf Life and Storage
| Feature | Description |
|---|---|
| Shelf Life | Typically 1-2 years from manufacture, consult supplier’s COA for details |
| Storage | Store at -20°C or below, sealed under inert atmosphere (argon or nitrogen), protected from moisture and light, avoid repeated freeze-thaw cycles |
Application
- Solid-Phase Peptide Synthesis (SPPS) as a building block for peptide chains
- Synthesis of peptides with turns, bends, or helical structures
- Peptidomimetic synthesis for molecules mimicking peptide structure/function
- Drug discovery targeting protein-protein interactions or specific peptide conformations
- Biomaterial design involving peptide-based materials with specific structure
- Studies on peptide folding and structure-function relationships
Key Characteristics
- Fmoc protection enabling base-labile N-terminal deprotection in SPPS
- Incorporation of Aib promotes helical conformations and peptide stability
- Proline introduces backbone kinks or turns
- Versatile for coupling with diverse amino acids to create varied peptides
- Soluble in organic solvents like DMF, DMSO, and acetonitrile
Citation
- Search for terms such as:
- “Fmoc-Pro-Aib-OH peptide synthesis”
- “Aib peptide helix” or “α-aminoisobutyric acid peptide conformation”
- “Proline peptide turn” or “proline-induced bend”
- “Helical peptide design with Aib and Pro”
- Utilize chemical literature databases like Reaxys, SciFinder, PubMed, and Google Scholar
- Check supplier websites and data sheets for technical details and application notes
Reviews
There are no reviews yet.