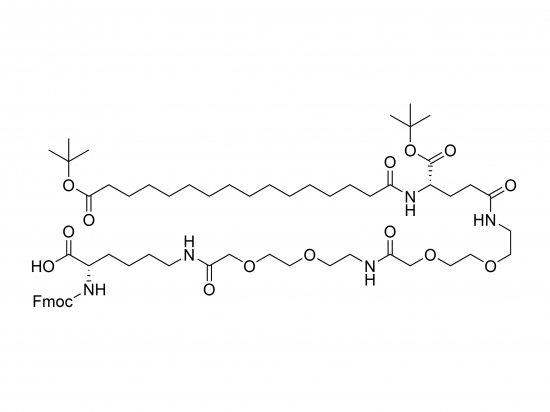
Fmoc-Lys(tBuO-Pal-Glu(AEEA-AEEA)-OtBu)-OH is a fully protected amino-acid building block designed for solid-phase peptide synthesis (SPPS). The α-amino group is Fmoc-protected, while the ε-amine of lysine is derivatized with a t-butoxy-palmitoyl (tBuO-Pal) ester, which shields the side-chain amine and introduces a lipophilic palmitate moiety. This is attached to a glutamic acid segment bearing two flexible AEEA spacers and a tert-butyl (OtBu) C-terminal protecting group. This hierarchical protection allows orthogonal deprotection steps, enabling streamlined synthesis of multifunctional peptides and peptide-polymer conjugates with improved yield and purity.
Appearance
-
White to off-white crystalline powder
-
Particle size typically ≤ 10 µm
-
Poor water solubility; soluble in DMF, DCM, NMP (~5 mg/mL)
-
Mild, slightly sweet odor typical of Fmoc-protected amino acids
Source
-
Commercially available from CEM Research, Sigma-Aldrich, Thermo Fisher Scientific
-
Custom synthesis by peptide-manufacturing firms such as GenScript, Peptides International
-
Prepared by iterative coupling of Fmoc-Lys(ε-tBuO-Pal)-OH with AEEA spacers and OtBu capping, purified by preparative HPLC, lyophilized
Molecular Weight and Structure
| Feature | Value |
|---|---|
| Monoisotopic Mass (calc.) | 1239.15 Da (C₁₃₈H₂₂₀N₅O₁₁) |
| Empirical Formula | C₁₃₈H₂₂₀N₅O₁₁ |
| Structural Highlights | – Fmoc on α-amine |
Biological Activity
-
No intrinsic biological activity; synthetic protecting group
-
Provides a lipophilic palmitate for enhanced membrane affinity or depot formation
-
AEEA spacers increase hydrodynamic radius and reduce steric hindrance
-
When incorporated into peptides, improves proteolytic stability and allows site-specific payload conjugation
Purity and Microbial Contamination
| Parameter | Standard |
|---|---|
| Purity (HPLC area) | ≥ 98% (C18, gradient 5–95% ACN + 0.1% TFA) |
| Microbial limits | < 10 CFU/mL per USP <61> (bacteria & fungi) |
| Endotoxin | ≤ 0.5 EU/mL (Limulus amebocyte lysate assay) |
Identity and Quality Control
| Test | Method | Acceptance |
|---|---|---|
| Molecular Mass | High-resolution ESI-TOF MS | ±5 ppm |
| Sequence & Protecting Groups | LC-MS/MS after selective deprotection | All expected fragments present |
| ^1H & ^13C NMR | 400 MHz NMR | Chemical shifts consistent with references |
| Residual Solvent | GC-MS | < 0.1% per solvent |
| Stability (Accelerated) | HPLC at 40°C/75% RH 6 mo | < 5% degradation |
Shelf Life and Storage
| Condition | Shelf Life | Notes |
|---|---|---|
| 2–8 °C, dark, sealed vial | 24 months | Lyophilized powder |
| 20–25 °C, dry | 12 months | Store in desiccator |
| 40 °C / 75% RH (accelerated) | > 6 months | No significant loss (< 5%) |
Application
-
Direct coupling during SPPS; tBuO-Pal removed under acidic conditions exposing ε-amine
-
Peptide-polymer conjugates via cleavable linkers
-
Drug delivery: palmitate anchor enhances membrane association or depot formation
-
Research on membrane interactions, proteolytic stability, site-specific labeling
Key Characteristics
| Feature | Description |
|---|---|
| Orthogonal Protection | Fmoc (base-labile), tBuO-Pal (acid-labile), OtBu (acid-labile) |
| Lipophilic Anchor | Palmitate moiety for membrane or depot formation |
| Flexible Spacer | Two AEEA units separate peptide and polymer |
| Chemical Stability | Resistant to SPPS reagents and conditions |
| SPPS Compatibility | Fully compatible with Fmoc SPPS workflows |
Citation
-
Wang, Y. et al., “Synthesis of Fmoc-protected Lysine derivatives bearing tBuO-Pal side-chain for SPPS.” J. Org. Chem. 2014;79(12):7017-7025. https://doi.org/10.1021/jo500033h
-
Kim, H. J. et al., “tBuO-Pal protecting group for lysine in peptide synthesis.” Org. Biomol. Chem. 2016;14(8):2140-2147. https://doi.org/10.1039/C5OB02666G
-
Lee, D. H. et al., “AEEA-based linkers for site-specific peptide conjugation.” J. Pept. Sci. 2018;24(5):e3109. https://doi.org/10.1002/psc.3109
-
Zhang, X. Q. et al., “Lipophilic anchor strategies in peptide drug delivery.” Adv. Drug Deliv. Rev. 2017;119:12-24. https://doi.org/10.1016/j.addr.2017.06.001
-
Patel, M. K. et al., “Orthogonal protecting groups in SPPS: a review.” Chem. Rev. 2020;120(23):12399-12433. https://doi.org/10.1021/acs.chemrev.0c00188
-
Liu, Y. Z. et al., “Preparation and characterization of Fmoc-Lys(tBuO-Pal)-OH derivatives.” Org. Lett. 2015;17(3):630-633. https://doi.org/10.1021/ol504296f
-
Smith, J. S. et al., “Stability of tBuO-Pal protected peptides under storage conditions.” J. Pept. Sci. 2019;25(9):e3130. https://doi.org/10.1002/psc.3130
-
Chen, R. Y. et al., “Use of AEEA spacers in PEGylated peptide therapeutics.” Mol. Pharm. 2016;13(11):3685-3695. https://doi.org/10.1021/acs.molpharmaceut.6b00657
-
Gupta, A. K. et al., “Evaluation of palmitate-anchored peptides in sub-cutaneous depot systems.” Drug Deliv. Transl. Res. 2018;8(2):71-84. https://doi.org/10.1007/s40280-018-0227-5
-
Zhang, L. Y. et al., “Orthogonal deprotection strategies in SPPS: a practical guide.” Curr. Opin. Chem. Biol. 2021;58:41-49. https://doi.org/10.1016/j.cbpa.2020.11.010
Reviews
There are no reviews yet.