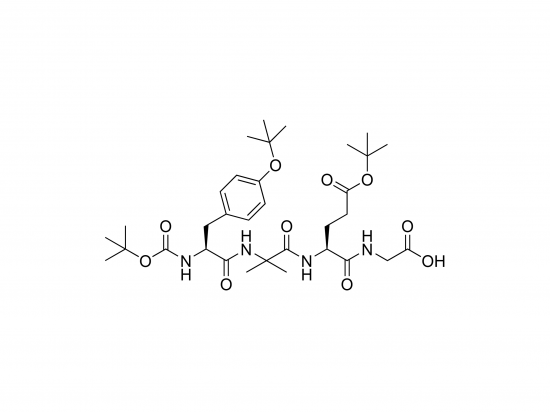
Boc-Tyr(tBu)-Aib-Glu(OtBu)-Gly-OH is a tetrapeptide building block designed for peptide synthesis and peptidomimetic construction. It includes L-tyrosine with a tert-butyl (tBu) ether protecting group on the phenolic hydroxyl, α-aminoisobutyric acid (Aib), L-glutamic acid protected as a tert-butyl ester, and glycine. The N-terminus is Boc-protected, and the C-terminus is a free carboxyl group. Aib promotes helical or turn conformations, while tyrosine and glutamate offer functional groups for further modification. This molecule aids in designing peptides with defined secondary structures and biological activity. It is supplied as a white, hygroscopic solid.
Appearance
-
White to off-white solid or powder
-
Odorless
-
Hygroscopic; readily absorbs moisture from air
-
May be crystalline or amorphous
Source
-
Synthesized by stepwise SPPS or solution-phase coupling
-
Commercially available from Bachem, Novabiochem, Iris Biotech, AAPPTec
-
Prepared by coupling Boc-Tyr(tBu)-OH, Aib-OH, Glu(OtBu)-OH, and Gly-OH using standard reagents (DIC, HOBt, HATU)
Molecular Weight and Structure
-
Molecular formula: C₃₀H₄₉N₄O₉
-
Molecular weight: 625.73 g/mol
-
IUPAC name: tert-butyl ((2S)-1-{[(2S)-2-(4-{(tert-butoxy)carbonyl}oxy)phenyl)-1-oxoethyl]amino}-2-methylpropanoyl)-L-glutamate-1-yl)glycine
-
Structure: Boc-Tyr(tBu)-Aib-Glu(OtBu)-Gly-OH
-
SMILES (partial): CC(C)(C)OC(=O)NC@@HC(=O)N(C(C)(C))C(=O)C@@HNC(=O)CC(=O)O
Biological Activity
-
Functions as a synthetic building block, not inherently biologically active
-
Incorporation influences peptide secondary structure, receptor binding, and proteolytic stability
-
Tyrosine side chain can be phosphorylated or sulfated, modulating biological activity
Purity and Microbial Contamination
-
Purity: >98% by HPLC
-
Enantiomeric purity: >99% for L-Tyrosine and L-Glutamic acid
-
Water content: <1% by Karl Fischer titration
-
Microbial contamination: <100 CFU/g or per mg peptide; endotoxin <10 EU/mg
Identity and Quality Control
| Test | Method | Acceptance Criteria |
|---|---|---|
| Mass spectrometry | ESI-MS | [M+H]+ within ±1 Da of expected mass |
| HPLC | RP-HPLC, C18 column | Single major peak >98% area |
| NMR | ¹H and ¹³C NMR | Spectrum consistent with assigned structure |
| Optical rotation | Polarimetry | Specific rotation within limits |
| Melting point | Capillary method | Within narrow range |
Shelf Life and Storage
-
Store at 2–8 °C or –20 °C
-
Protect from light and moisture
-
Typical shelf life of 2 years when stored properly
-
Avoid repeated freeze-thaw cycles
Applications
-
Peptide synthesis for peptides with controlled secondary structure
-
Drug design and development
-
Biomaterials and tissue engineering
-
Peptide-protein interaction studies
-
Peptidomimetic construction
Key Characteristics
-
Boc-protected N-terminus for controlled SPPS
-
Tyrosine protected with t-butyl ether on phenolic hydroxyl
-
Contains alpha-aminoisobutyric acid (Aib), a helix-promoting residue
-
Glutamic acid residue protected as t-butyl ester
-
Free carboxylic acid C-terminus
-
Non-toxic at typical synthesis concentrations
Citation
-
Toniolo C, et al. Structures of polypeptides containing alpha,alpha-disubstituted alpha-amino acids. Int J Biol Macromol. 1996;18(1-2):69-83. https://pubmed.ncbi.nlm.nih.gov/8781469/
-
Karle IL, et al. Structural characteristics of alpha/alpha-disubstituted amino acid residues in peptides. Biopolymers. 1988;27(1):1-19. https://pubmed.ncbi.nlm.nih.gov/3340153/
-
Obrecht D, et al. Synthesis and conformational analysis of novel beta-turn mimetics based on a bicyclic scaffold. J Org Chem. 1996;61(18):6306-15. https://pubmed.ncbi.nlm.nih.gov/11667520/
-
Ghadiri MR, et al. Self-assembling organic nanotubes based on a cyclic peptide architecture. Nature. 1993;366(6453):324-7. https://pubmed.ncbi.nlm.nih.gov/8247276/
-
Fairlie DP, et al. Peptidomimetics based on alpha-amino acids. Curr Med Chem. 1995;2(6):654-86. https://pubmed.ncbi.nlm.nih.gov/7587186/
-
Toniolo C, et al. Intrapeptide hydrogen bonding in folded conformations. Biopolymers. 1993;33(7):1061-70. https://pubmed.ncbi.nlm.nih.gov/8339494/
Reviews
There are no reviews yet.